Big
thin bubbles are art
Unless you have designed the bubbles, they are mistakes,
not art. Even when designed, they are
delicate and when broken are very sharp.
So, they cannot be sold or used as they are even by yourself.
Bubbles within the
glass in a plate.
fusing101.files.wordpress.com
People
frequently make the suggestion that the bubble should be broken and the cavity
filled with frit. Of course this can be
done, but almost always appears a fix rather than a design choice.
The more
important thing is to learn the cause so it can be prevented in the
future. Bubbles can be between layers or
from underneath the whole piece.
Bubbles
between the layers of glass are usually the result of inclusions or layup and
firing rates. Anything which holds the
upper layer above the lower one has the potential to induce bubbles. Most often, with a bubble squeeze, these are
relatively small and are 2mm or more thick.
These may be acceptable or seen to be unsightly, but are not
dangerous. The bubbles can become large
and/or thin with high temperatures or fast rises in temperature. Be sure to have a good bubble squeeze, and a
moderate (ca. 300°C) rise in temperature from there.
Bubbles can
also rise between the shelf and the glass.
This happens most often when firing single layers above a low
temperature tack fuse.
A single layer piece
with large, burst, healed and emerging bubbles.
www.warm-glass.co.uk
It can also
occur when there is either debris between the glass and shelf, or when there is
a depression in the shelf. Both these
cases allow air to remain trapped between the shelf and the glass. Slower rates of advance and bubble squeezes
can help reduce these, but the shelf needs to be checked for debris and high or
low spots.
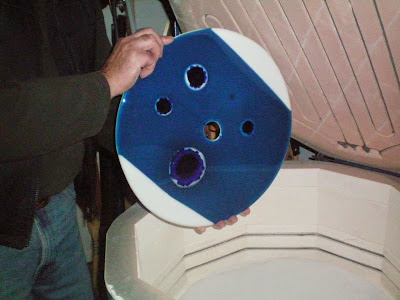
The piece
below is disfigured by the random bubbles at the left and in the centre of an
otherwise acceptable platter.
fusedglass.files.wordpress.com
Evaluate your
pieces before you declare a single or series of large thin bubbles art. Of course, you should play around with the
piece to learn from the mishap. You can
use the pieces of it in other projects.
But unless it is truly exceptional, it is a mistake, not art.
All myths have an element of truth in them otherwise they would not persist.
They also persist because people listen to the “rules” rather than thinking about the principles and applying them. It is when you understand the principles that you can successfully break the “rules”.