Using Embolic Glass Microspheres to Target Chronic Disease
By Sierra Kucko
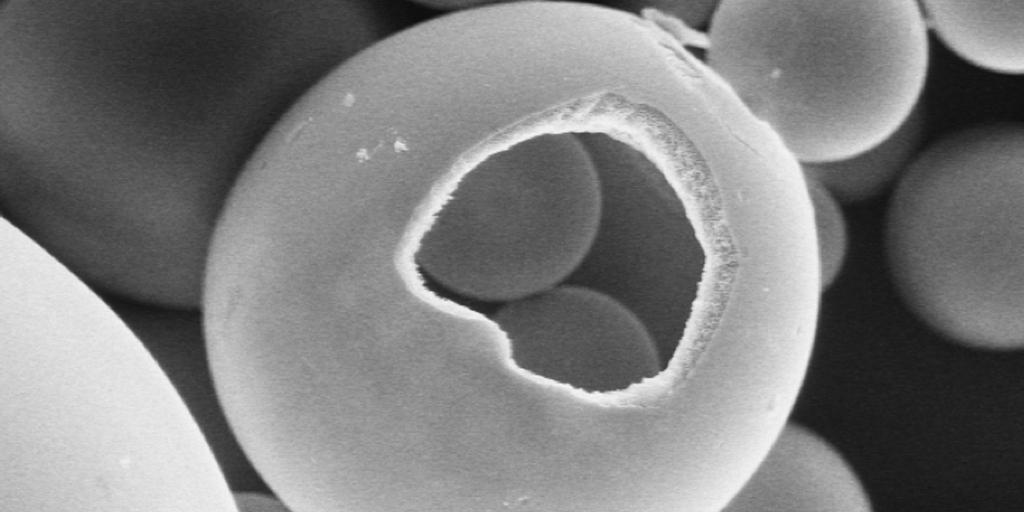
Glass Microspheres: The Tiny Superheroes of Glass Form Factors
Two main factors contribute to the properties of glass: composition and form factor. While emphasis is often placed on glass composition, the form factor is arguably equal in importance. Glass in the form of microspheres has permeated various industries, ranging from aerospace to medical sectors. MO SCI specializes in the production of precision glass microspheres that have become invaluable to these industries, and their usage is ever-evolving. Whether it be controlling gaps for adhesive bondline spacing, improving the visibility of road markings, or drug delivery devices, glass microspheres fit the bill.
Targeted Treatment with Embolic Glass Microspheres
Biocompatible (and in some cases biodegradable) microspheres are especially appreciated for medical applications, such as transarterial embolization (TEA) or musculoskeletal (MSK) embolization.1,2
TEA refers to the blockage of blood supply, which may sound like a bad thing, but in many cases, these are lifesaving procedures. For example, a substantial driver for this technology is cancer treatment. One way to combat a tumor or abnormal tissue growth is to cut off its blood supply, which can be achieved through the precise application of appropriately sized microspheres to occlude the fine vasculature ‘feeding’ it.1
Similarly, MSK embolic microspheres are sought after to prevent the abnormal overgrowth of blood vessels, a consequence of chronic inflammation. This kind of inflammation is part of a pathological loop, whereby the inflammation promotes the formation of new blood vessels that in turn, can feed nerve growth and contribute to chronic, debilitating pain.2 Microsphere embolization can therefore be used as a pain management tool, as well. For applications with this level of weightiness, the microsphere size is a chief feature.
Together with the form factor, glass microspheres can be tailored through their composition. First and foremost, any implantable glass must be compatible with the body. Ancillary to this, the composition can be altered to offer additional functionality. Using TAE as an example to put this concept into context, the composition of glass used in this type of application is unique and important.
TAE is a procedure utilized by interventional radiologists. Interventional radiology (IR) is the diagnosing and/or treatment of cancer and other conditions while avoiding major surgery. To achieve this, small tools such as needles, catheters, or wires are utilized in conjunction with radiation like MRI, ultrasound, etc. to apply treatment precisely to the tissue site.1–3 Personalization and optimization of outcomes is a clinical challenge of any medical intervention, making the accurate delivery and distribution of the embolic particles in real-time indispensable.
Due to the use of radiation to guide the placement, the embolic particle should be radiopaque (opaque to radiation) to ensure that guided delivery to the site can be realized. Compared with glass, this radiopacity is lacking or more difficult to achieve in microspheres derived from other material types.
Partner with MO SCI for Precision Glass Microspheres
Each application of glass is unique and therefore may require unique chemistries and form factors. Glass microspheres are becoming increasingly popular, since their form factor alone may improve the function of the glass (depending on the application) when compared to their powder or frit counterparts.
For applications that require precise microsphere size and composition, it is important to turn to trusted experts. MO SCI produces a wide range of glass microspheres in a variety of chemistries to suit nearly any need. Contact us today to learn how glass microspheres may be beneficial for your application.
References
Pérez-López A., et al. (2022). Embolization therapy with microspheres for the treatment of liver cancer: state-of-the-art of clinical translation. Acta Biomaterialia. https://doi.org/10.1016/j.actbio.2022.07.019
Gremen E., et al. (2022). Safety and efficacy of embolization with microspheres in chronic refractory inflammatory shoulder pain: a pilot monocentric study on 15 patients. Biomedicines. https://doi.org/10.3390/biomedicines10040744
Kishore S, et al, (2021). Transarterial embolization for the treatment of chronic musculoskeletal pain: a systematic review of indications, safety and efficacy. ACR Open Rheumatology. https://doi.org/10.1002/acr2.11383