Wednesday, 7 September 2022
Silberschnitt Runners
Hazards of Flux Fumes
Risks are assessed as acute and chronic. Acute means immediate reaction. Chronic means the effects are cumulative and may take years to appear.
Composition of Flux
Zinc Chloride Risks
http://www.inchem.org/documents/ukpids/ukpids/ukpid86.htm#:~:text=Toxicity%20Zinc%20chloride%20is%20corrosive,anorexia%2C%20fatigue%20and%20weight%20loss.
Ammonium Chloride Risks
· Skin Contact: Immediately flush skin with water and disinfectant soap and use an emollient on irritated area.
· Eye Contact: Rinse eye(s) with water for at least 15-20 minutes. Protect unexposed eye.
· Ingestion: Rinse mouth thoroughly with water. Do NOT induce vomiting.
· Inhalation: Move to fresh air and administer artificial respiration if needed.
https://www.msdsonline.com/2017/05/05/chemical-spotlight-ammonium-chloride/#:~:text=Exposure%20to%20Ammonium%20Chloride%20is,particulate%20dispersed%20in%20the%20air.
Hydrochloric Acid Risks
Acute Effects:
Chronic Effects:
https://www.epa.gov/sites/production/files/2016-09/documents/hydrochloric-acid.pdf
Phosphoric
Acid Risks
Inhalation — Seek fresh air and immediate medical attention.
Eye Contact — Remove contact lenses if present. Immediately flush eyes with plenty of water for at least 15 minutes and get medical attention.
Skin Contact — Wash skin with soap and water. Cover any irritated skin with an emollient. Seek medical attention.
https://www.msdsonline.com/2015/06/17/phosphoric-acid-safety-tips/
Citric Acid
https://sciencing.com/hazards-citric-acid-8165149.html
Remember that this irritation is equivalent to squirting lemon juice into your eye. It is not a chronic risk.
Hydrobromic Acid (HBr)
https://web.stanford.edu/dept/EHS/cgi-bin/lcst/lcss/lcss47.html#:~:text=The%20aqueous%20acid%20and%20gas,gas%20can%20produce%20severe%20burns.
Precautions to be taken by glass workers
Wednesday, 31 August 2022
Choosing a Name for Your Business
Personal Name
Many creative sole traders use their own name as their business name. It is easy and often creates the personal connection sought for by buyers. Using your own name gives your business a more arts oriented and personal touch. Sometimes people are already ‘known’ under their own name prior to beginning the formal business, e.g., Amanda J Simmons.Initials
Some designers use their initials or attach their discipline to their name or initials to add some clarity to what they do, e.g., MJM Ceramics. Others prefer a business name, so that it is less personal and not based around one individual. This can appear as more professional and allows the business to grow beyond the individual.Reflect on your vision, values, and passions
You have already pulled out these things in looking at your life goals and vision. Now you need to apply them to a name search. A method that may help is to write down ten important descriptive words about these values, passions, and your vision. This is a starting point for thinking about the best words for the business. These words will probably not come immediately. You might want to involve others in a kind of brainstorming to develop a group of key words. You need to consider how this name will look in the future as your business develops.The name needs to reflect you and your market.
“A good business name reflects who you are and which ideal clients you want to attract.” It is the introduction to the business. It is the first thing they see, even before you. So, what you do and who you do it for is important to selecting the name. The name creates an image in customers’ heads. Do you want to be factual or do you want to create a bit of mystery? To help with this selection you need to remember your niche market and what you do and for whom as defined earlier. Review the business specialism - what you want to be known for - and your ideal clientsInspiration sources
You do not have to be factual in the name and you can get inspiration from a number of sources. Favourite songs, places, people, films, etc., can all be sources. Brainstorm with others for names around your values, vision, passions, your specialism, your ideal clients. You can also get ideas from the business names your competitors or role models use. You need to record these ideas. This can be written, but an image or sketch can be even more useful. A mood board or mind map can be helpful too. A logo idea often comes with considering a name. You should strive to have about five good, creative names for your business to make a choice. Not all the names will be available. There are also online business name generators that can help focus your ideas.Check the potential names
You need to make sure of several things before you settle on the name.This is important in online searches as they are the most commonly used method of finding businesses. You need to avoid quirky expressions, and names with common variations (e.g., is it Mc, Mac or M’)
Almost as important as spelling is being able to pronounce the name in different dialects or languages. This is a worthwhile consideration as exemplified in car model names.
This goes together with the verbalisation of the name. If it is easy to say, it probably is easy to remember and so be searched for without difficulty.
Try to ensure your name does not mean something offensive in another language. Even if you are not operating internationally (just now), your name will be visible throughout the world.
This is harder than it sounds, but you want the business name to be acceptable in a generation’s time. This means that you avoid names that are “now”.
Companies House in the UK gives a list of sensitive and prohibited words on its website. This is most useful even if you do not intend to register with them. It helps you avoid current and future difficulties in a business name. There are equivalents in other countries. Is the name the same - or nearly so - as a registered trademark? There is more information about registered trademarks on the Intellectual Property Office website (a UK resource).
You can use google to check on the existence of the name as a business one. The Companies House website also has a free facility to check on name conflicts of registered companies. Check to see if your proposed business name is available as a web address. Also check on Facebook, Twitter, and other social media sites. It is best to have the same name and address across all the web and social media sites.
Wednesday, 24 August 2022
Accuracy in Following the Cartoon
A question arose on Facebook
that resolved itself around the need (or not) to accurately follow the cartoon.
The question itself was whether a pictured piece should be re-cut. It was a
relatively complicated piece, which in its current state left two significant
gaps between the glass and the cartoon line.
The vast majority of responses, was along the lines of don’t worry, just adjust the neighbouring pieces to fill the gaps. (“enjoy yourself” was another theme).
To follow the majority view - make adjustments in other pieces to allow the problem piece to fit - encourages bodge. It does not encourage development in the skills of the craft. It does not encourage long term enjoyment. It pushes improvement well into the future.
The cartoon is the design in line form. Its purpose is to control the construction of the piece. It is important to follow the cartoon to express the design. This means striving to cut and fit the glass to the cartoon as accurately as possible. Line is important in stained glass, as everyone knows. If the glass is not fitted accurately, the lines will not flow according the cartoon and the design.
This is not to say the cartoon cannot be modified. It is important to look at the cartoon in detail once you have been attracted by a design. You need to look at each piece and determine its difficulty. Can I cut it? Is the design overly complicated? How might I modify the cartoon to make easier to cut pieces, and still maintain the overall design? Ensure the cartoon is drawn with accurately thin lines for the form of the craft – copper foil or leaded glass, and fused glass. Revise your cartoon to respond to the answers to this review of it.
Whatever method you use to score and break your glass, fit the piece to the cartoon as accurately as possible to avoid excess work later. The grinder is there to make fine adjustments to the cut piece so it will fit the cartoon. If the glass fits snugly within the cartoon lines, there should be little, or no, alteration required to the cartoon during the rest of the construction.
This is not about the methods you use during the construction of a piece. That is open to many approaches to achieve the same end. It is about an approach to the craft. In particular, it is about how the cartoon is used to guide the work. If the detail of the cartoon is optional, the final result may vary from the original conception significantly.
Craft development is
about striving to perform the actions as accurately as possible throughout the
work. The increased skill level that this develops, provides long-term
enjoyment. And each completed piece will
give a feeling of accomplishment.
Wednesday, 17 August 2022
Hake brushes
Hake (ha-kay) brushes are made from goat's hair. Their advantage over other brushes for applying kiln wash is that they hold a lot of liquid. Proper ones made from joined bamboo work better than the ones with flat handles.

Traditional Japanese hake brush
People often note that these brushes
tend to shed hairs. The solution to stray hairs (given to me in a Bullseye
workshop) is to invert the new brush and apply super glue at the point where
the hairs emerge from the handle. This holds the hairs in place. It will
work on flat handles too.

Inexpensive goat's hair brushes of the hake style.
As can be seen by comparison, there are fewer hairs in these.
Wednesday, 10 August 2022
Kiln wash application with a brush
Kiln wash is
applied thinly in a 1:5 powder to water mix to shelves and moulds. The object is to get a complete coverage with
a smooth surface.
To ensure full coverage painting four coats is sufficient for excellent coverage. The kiln was should be applied in four directions – horizontal, vertical, and each diagonal. This ensures any gaps in one coat will be covered by the others. A broad brush that holds a lot of liquid provides good coverage. A hake brush is ideal. The brush should be held almost vertical with the ends of the bristles only touching the surface.
 |
| A traditional Japanese hake brush |
There is no need to dry each coat before applying the next. It is not like painting your wall. All coats can be applied one directly after the other. No drying between coats is required. In fact, earlier dried coats tend to make the application clumpy and streaky.
Some people advocate a fifth coat. I don’t know what the fifth coat is for. What direction other than the four cardinal ones can there be? It maybe it is insurance that the surface is coated evenly. This can be checked visually. The kiln washes used for glass are routinely coloured. If the shelf shows unevenly through the kiln wash, a little more needs to be brushed onto the more thinly coated area.
It is possible to smooth the
kiln washed surface once the kiln wash has a dusty surface – it does not have
to be completely dry – you can put a piece of paper between the shelf or mould
and your hand. Gently rub the surface to
get a really smooth finish to your kiln washed shelf.
https://glasstips.blogspot.com/2009/08/applying-kiln-wash.html
https://glasstips.blogspot.com/2009/08/smooth-kiln-wash-on-shelves.html
Wednesday, 3 August 2022
Vitrigraph Pots from Refractories
Vermiculite
 |
| The screws at the left side of the box are omitted in the drawing, but are required |
 |
| The screws at the left side of the box are omitted in the drawing, but are required |
Refractory fibre
Wednesday, 27 July 2022
Softening the Tack Profile
You can do either.
Sunday, 24 July 2022
Phase Separation and Crystallization in Glass
From the Mo-Sci Corporation Blog:
Posted by Krista Grayson
While historically a source of problems for glass producers, the phenomenon of phase separation is now known to offer advantages in the production of certain materials such as glass ceramics and porous glasses. Whether desirable or undesirable, understanding and controlling phase separation during the glass manufacturing process is crucial. In this article, we explore the basics of phase separation and how it can be manipulated to create advanced materials for various applications.
What is phase separation?
In physics and chemistry, the word “phase” refers to a region of a material that is chemically uniform and physically distinct. Phase separation, which typically occurs in liquids, is where a homogeneous mixture separates into two or more of these phases. For example, a mixture of water and oil at room temperature will naturally “phase separate” into a distinct phase consisting of pure oil, and another consisting of pure water. We can say that such a mixture is “immiscible.”
The morphology of this phase separation can vary depending on the relative concentration of both components. If the mixture is predominantly water, the oil phase will take the form of distinct (or “discontinuous”) droplets dispersed throughout an interconnected (or “continuous”) water phase. If the mixture is predominantly oil, the opposite will take place. At roughly equal proportions of oil and water, each phase will tend to be continuous.
Phase Separation in Glass
Phase separation commonly occurs in glass melts. Borosilicate glass – which contains both silica and borate as network formers – is a well-studied example.1,2
Unlike our water/oil example, phases in glass melts are not necessarily chemically pure. Borosilicate glass, for example, will typically undergo phase separation into a “borate-rich” phase and a “silica-rich” phase, with both phases containing different proportions of each network former. In addition, the morphology of separated phases in glass can vary. While it is possible for droplet-like phases to form via classical nucleation and growth, spontaneous “spinodal” phase separation can result in the formation of intertwined tendril-like continuous phases.3
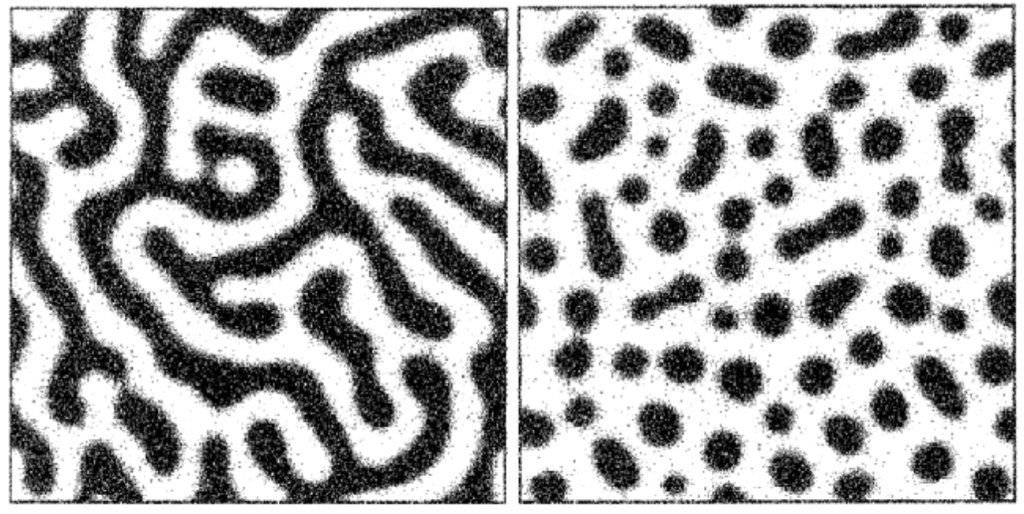
This phase separation, which occurs at high temperatures in the molten glass, persists and “freezes in” when the glass is cooled into a solid. If both phases are vitrifiable, they may form glasses after cooling (this is called a glass-glass phase separation). However, if one phase is prone to crystallization, the mixture can cool into a glass-crystal phase-separated solid.5
Phase separation in glasses was long seen as undesirable – and for many applications, it still is.6 The existence of different phases modifies the physico-chemical properties of glass melts, making it difficult to mold and reduce the quality of the final glass.
The physics of phase separation in glass-forming materials is complex, and even today the specifics are subject to intense debate.7 However, glass manufacturers nonetheless determined ways of avoiding or minimizing phase separation during glass manufacturing.
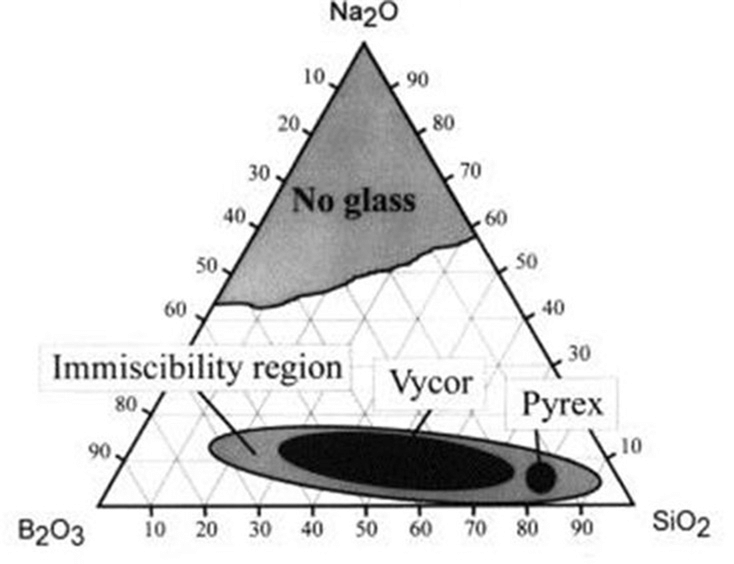
Typically, this is achieved by tailoring the composition of glass melts, with phase separation only occurring for specific compositions. In a Na2O–B2O3–SiO2 glass system, for example, the following ternary phase diagram shows the immiscibility region in which phase separation will occur.
Phase separation (and subsequent crystallization) can also be controlled by the addition of glass modifiers, and by varying heat treatment and cooling rates.9
Controlling and Exploiting Phase Separation in Glass
Note that within the immiscibility region in the diagram above, two common commercial glass compositions are labeled. Indeed, it’s now understood that phase separation offers advantages in certain applications. Today, heterogeneous phase-separated glasses cover a broad range of commercial applications, including Pyrex®, Vycor® opal glass, porous glass, and glass ceramics.
Glass-ceramics are a class of polycrystalline materials that share many properties with both glasses and ceramics, ideally providing the moldability of glasses with various special properties (such as high strength) of ceramics. Glass-ceramics are produced by the formation of crystal phases within an amorphous base glass (i.e., crystal-glass phase separation). Engineering glass-ceramics depends on controlling crystallization within the base material.10
Another application of controlled phase separation is in the production of porous glasses. Porous glasses are typically high-silica glasses that contain pores with a specific size distribution, ranging from angstrom to millimeter scales. Porous glasses are commonly produced from phase separation of alkali borosilicate glass, in which the mixture undergoes spinodal phase separation following heat treatment to yield two continuous phases.11 Following phase separation, the alkali-rich borate phase can be dissolved in acid and removed from the solid. This leaves a highly pure and porous silica glass “skeleton.”
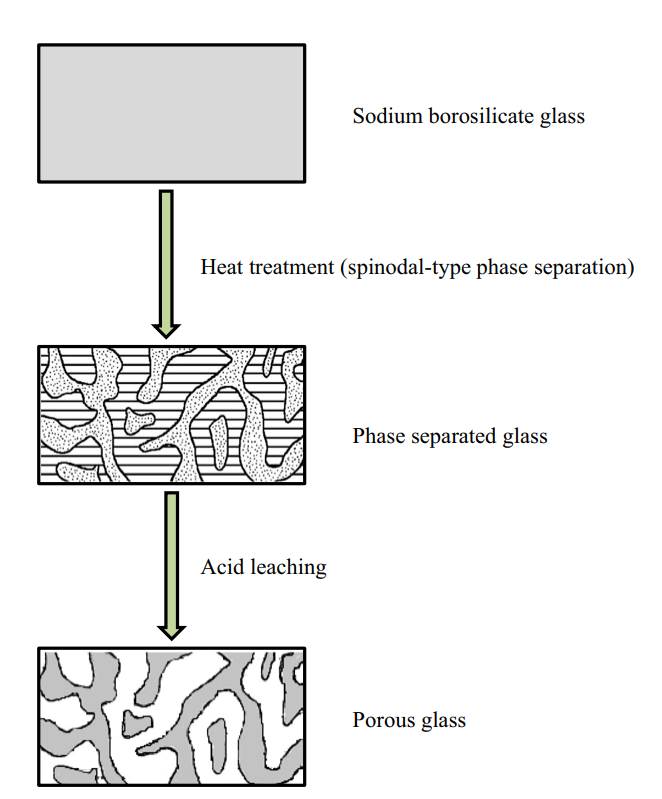
Porous glass exhibits improved mechanical and thermal stability compared to ordinary bulk glass, making it a popular alternative to fused quartz which is comparatively difficult to form. Other applications make use of the pores themselves: such as filitering materials, catalyst supports, and targeted drug delivery.12–16 Mo-Sci is a world-leading provider of advanced glasses for healthcare, electronics and engineering applications. We offer a range of glass-ceramic seals and porous glass solutions, as well as providing custom solutions for virtually any glass application. Contact us for more information.
References and Further Reading
- Charles, R. J. Phase Separation in Borosilicate Glasses. Journal of the American Ceramic Society 47, 559–563 (1964).
- Möncke, D., Ehrt, D. & Kamitsos, E. Spectroscopic study of manganese-containing borate and borosilicate glasses: Cluster formation and phase separation. Physics and Chemistry of Glasses – European Journal of Glass Science and Technology Part B 54, 42–51 (2013).
- Bergeron, C. G. & Risbud, S. H. Introduction to Phase Equilibria in Ceramics. (American Ceramic Society, 1984).
- Gebauer, D., Kellermeier, M., Gale, J., Bergström, L. & Cölfen, H. Pre-nucleation clusters as solute precursors in crystallisation. Chemical Society reviews 43, 2348–2371 (2014).
- Schuller, S. Phase separation in glass. (2018).
- Morey, G. W. The Properties of Glass. (Books on Demand, 1954).
- Da Vela, S. et al. Interplay between Glass Formation and Liquid–Liquid Phase Separation Revealed by the Scattering Invariant. J. Phys. Chem. Lett. 11, 7273–7278 (2020).
- Bartl, M. H., Gatterer, K., Fritzer, H. P. & Arafa, S. Investigation of phase separation in Nd3+ doped ternary sodium borosilicate glasses by optical spectroscopy. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 57, 1991–1999 (2001).
- Liu, S., Zhang, Y. & Yue, Y. Effect of cooling rate on crystallization in an aluminophosphosilicate melt. Physics and Chemistry of Glasses – European Journal of Glass Science and Technology Part B 52, (2011).
- Control of nucleation in glass ceramics | Philosophical Transactions of the Royal Society of London. Series A: Mathematical, Physical and Engineering Sciences. https://royalsocietypublishing.org/doi/10.1098/rsta.2002.1152.
- Hasanuzzaman, M., Rafferty, A., Sajjia, M. & Olabi, A.-G. Production and Treatment of Porous Glass Materials for Advanced Usage. in Reference Module in Materials Science and Materials Engineering (Elsevier, 2016). doi:10.1016/b978-0-12-803581-8.03999-0.
- Hammel, J. J. & Allersma, T. United States Patent | Thermally stable and crush resistant microporous glass catalyst supports and methods of making. 341–341 (1975).
- Jungbauer, A. Chromatographic media for bioseparation. Journal of Chromatography A 1065, 3–12 (2005).
- Sotomayor, P. T. et al. Construction and evaluation of an optical pH sensor based on polyaniline-porous Vycor glass nanocomposite. in Sensors and Actuators, B: Chemical vol. 74 157–162 (2001).
- Takahashi, T., Yanagimoto, Y., Matsuoka, T. & Kai, T. Hydrogenation activity of benzenes on nickel catalysts supported on porous glass prepared from borosilicate glass with small amounts of metal oxides. Microporous Materials 6, 189–194 (1996).
- Using Porous Glass Microspheres for Targeted Drug Delivery Mo-Sci Corporation. https://mo-sci.com/porous-glass-microsphers-targeted-drug-delivery/.
 Share
Share Tweet
Tweet Share
Share















