 |
| Credit: Artefact Contemporary Craft Fair at Vessel Gallery |
Wednesday, 30 March 2022
Attending Craft Fairs is Important
Feedback
Raise your Profile
Maintain Contacts
New Clients
Events Can Help Online Sales
Physical Presence
Deadlines
Sunday, 27 March 2022
Vitrification: The Workhorse of Nuclear Waste Management
Vitrification: The Workhorse of Nuclear Waste Management
Posted Krista Grayson on Jun 18, 2019

Nuclear power has been used to produce electricity for over 60 years, and is responsible for around 16% of the electrical energy produced worldwide. This percentage is likely to increase, as countries move away from fossil fuels and require a source of energy to make up the shortfall this will cause.
With more reactors coming online, more fuel will be used, and this presents a problem: there is no conclusive method to handle the spent fuel. Vitrification (the transformation into glass) of nuclear waste is one established solution, but there is still plenty of room for improvement in this method.
The issue of nuclear waste
A key problem with nuclear power is what to do with the waste products generated when the fuel is completely spent. Although it is no longer useful in power generation, this waste is still very dangerous if not stored correctly.
The most problematic waste, called ‘high level’ nuclear waste (HLW), is highly radioactive, has an extremely long half-life and requires cooling and containment due to the elemental decay, which gives off heat and radiation.
Additionally, some radioactive isotopes such as Tc-99, Se-79, and I-129 are mobile in water, which requires further measures in order to reduce their ability to move into the groundwater. Secondary waste streams can also present issues as this waste can contain large amounts of molybdenum and noble metals.
Safely storing nuclear waste through vitrification
One method of long term storage and disposal involves the processing and transformation of the spent fuel into a glass, a technique known as vitrification. It has been used for HLW immobilization for over 40 years in most countries that have a nuclear power program, including France, Germany, Belgium, Russia, UK, Japan, and the USA.
Glass is desirable as a long-term storage form as it is a relatively insoluble, compact and solid. In this form it is easier to store and handle, saving space and reducing cost. Glass also possesses high chemical durability, allowing it to remain in a corrosive environment for thousands or even millions of years without failing. While glass is often thought of as a fragile material, a properly treated block of borosilicate glass is incredibly resilient.
How vitrification works
The process of vitrification is quite simple but can be difficult to execute. First, the waste is dried, then heated to convert the nitrates to oxides. Glass-forming additives are added to the waste material and heated again to around 1000 °C. The molten liquid is poured into a suitable containment vessel to cool and form the glass. Once solidified, the final vitreous product has incorporated the waste contaminants in its macro- and micro-structure, and the hazardous waste constituents are immobilized.
The two main types of glass currently used to immobilize nuclear waste are borosilicate and aluminophosphate glasses. Both of these materials allow high waste loadings and can immobilize large amounts of actinides. Borosilicate glasses can accommodate up to 7.2 mass pct of PuO2 for example.
Advantages and limitations of nuclear waste vitrification
Although vitrification is often the preferred method of waste storage, there are some drawbacks to the current techniques, both with the necessary setup and materials used. Vitrification has a high initial investment cost, high operational cost and complex technology requiring qualified personnel.
This makes it most economically viable in locations where relatively large volumes of radioactive waste with stable composition are available, such as HLW from nuclear power plants.
Unfortunately, the current generation of glasses cannot handle large amounts of MoO3 and noble metals that are produced from secondary waste streams. These compounds are poorly soluble in borosilicate glasses and this limits the amount of waste that can be loaded into the material, increasing process time and material costs.
New materials to process molybdenum-rich nuclear waste
It is clear that vitrification is of vital importance for the long term success of nuclear power. Mo-Sci has been working on new types of glass that can immobilize a higher percentage of waste, as well as methods within the processing that can speed up the vitrification.
This includes an iron phosphate waste form able to contain 40 wt% of the simulated molybdenum-rich nuclear waste. This vitrified nuclear waste is prepared by melting the mixture of simulated waste components and iron phosphate glass additives in a commercial-scale cold crucible induction melter (CCIM). When the chemical durability of the waste form was measured, it was found to be as good as or better than that of borosilicate glass.
The CCIM melting technology can also process waste forms faster, safer and at a lower cost than other melting technologies as it removes the metal electrodes that directly contact the molten glass and refractory used to contain the melt. This novel CCIM-processed iron phosphate waste form could drive large savings in time and money in the industry’s need to remediate nuclear waste, and make nuclear power an even more attractive option for the future.
See our article on iron phosphate waste forms for more information on these new developments.
References
- Thompson, L. (2010) Vitrification of Nuclear Waste http://large.stanford.edu/courses/2010/ph240/thompson2/
- Criscenti, L. et al. (2013). An international initiative on long-term behavior of high-level nuclear waste glass. Materials Today, 16 https://doi.org/10.1016/j.mattod.2013.06.008
- Ojovan, M. I., & Lee, W. E. (2011). Glassy Wasteforms for Nuclear Waste Immobilization, 42 https://doi.org/10.1007/s11661-010-0525-7
- Ojovan, M. I. (2007). Glasses for Nuclear Waste Immobilization, WM’07 Conference. https://www.researchgate.net/publication/267700284_Glasses_for_Nuclear_Waste_Immobilization/download
- Cheol-Woon, K. (2018) Iron Phosphate Waste Forms for Nuclear Waste Disposal https://mo-sci.com/iron-phosphate-nuclear-waste-disposal
Tuesday, 22 March 2022
Selling online
 |
| Credit: 48HoursLogo.com |
Images
Get and maintain interest
Provide information
Purchase
What’s for sale
Develop trust
Buying and delivery
Sunday, 20 March 2022
Are Electric Furnaces the Future of Glass Manufacturing?
Are Electric Furnaces the Future of Glass Manufacturing?
Posted Krista Grayson on May 16, 2019
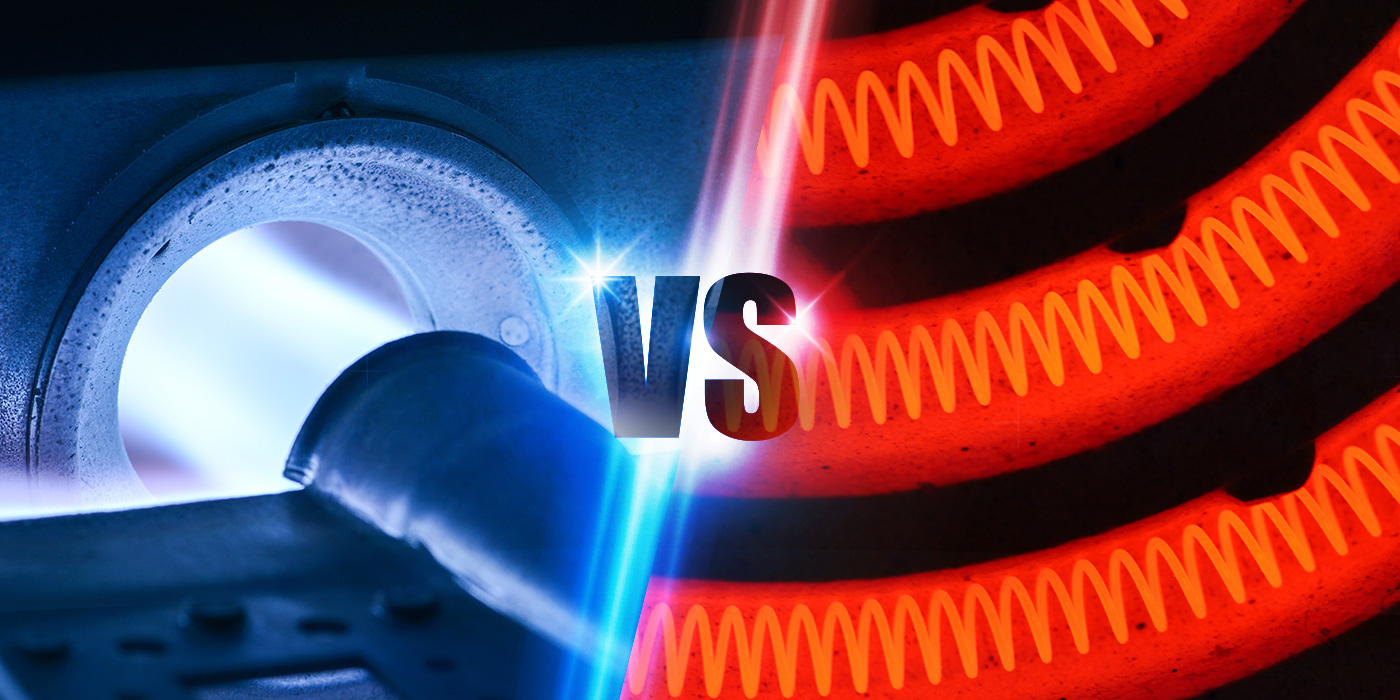
Overview of glass production
Glass production is typically energy-intensive. Glass furnaces may reach 1300-1550 ºC for the melting and refinement of the raw materials, depending on the formulation required.
Natural gas and electricity are the main energy sources, however historically, the glass industry has favored gas because it is an established technology, with low price, high purity, ease of control and the fact that there is no requirement for storage facilities. Gas furnaces have long life-times, on average over 12 years and sometimes up to 20 years.
Until recently electric glass melting furnaces have been used for specialty glasses, and particularly glasses with significant volatile constituents such as fluoride opal glasses, borosilicates and lead crystal. Interest is growing in extending its use through the industry.
Electric glass furnace production
The most effective method of electric glass production is to use electrodes immersed in the glass either as electric boosting (providing 5-20 % of total energy input) or all-electric melting. The immersed electrodes are connected to a power supply and transformer, to pass an electric current through the glass.
In all-electric furnaces, the melting energy comes from the electrodes (joule heat), with a gas burner being used for the initial start-up, or as an emergency heat source. These furnaces mainly operate ‘cold top’, where the raw material is distributed evenly over the melting surface of the glass, forming an insulating ‘batch blanket’. Melting and refining take place in one vertical process, with glass being drawn through a throat at the bottom of a deep melting tank.
Advantages of electric melting
Electric furnaces offer several advantages over gas furnaces. For example, they have very low direct emissions of CO2, thermal NOx or SOx emissions. With pressure to reduce emissions coming from both customers and legislation, this is a significant benefit. While it is possible to improve conventional gas furnaces to reduce emissions, this can result in more complex technology that results in additional maintenance, the use of non-environmentally friendly chemicals, and limitations to equipment lifespan.
Another benefit is that heat losses from electric furnaces are much lower. The thermal efficiency of gas furnaces peaks at around 45%. This means more energy is lost as heat than is used to convert the raw materials to molten glass. Heat losses occur from the superstructure of the furnace and in the residual waste gases, even if heat recovery systems are used. In contrast, the electrical approach means that the melting energy is transferred directly into the glass. Thermal efficiency can be over 70% even in a small electric furnace and can reach 85% in a large electric furnace.
All-electric furnaces are also more energy efficient than gas-fired furnaces; they use around 35% less energy. The difference in energy efficiency is particularly important for small furnaces. As furnace size decreases, the energy efficiency of electric furnaces remains very high, whereas the efficiency of gas furnaces drops dramatically and can be less than 20%.
Electric boosting can be a highly effective way to reduce overall energy consumption. It also means that energy release can be highly focused, helping to determine conditions in the glass bath. In some cases, a well-designed boost system can improve glass quality homogeneity, seed and stone losses. In contrast, in gas furnaces, where focused energy release is not possible, imprecise temperature profiles can be created in the glass.
A key advantage of the cold-top electric furnace is that everything that goes into the batch stays in the glass, aside from the gases released from the melting process, which permeate out through the batch blanket. Losses of batch constituents such as fluorine, boron, lead, various volatile refining agents and other constituents are almost eliminated.
Disadvantages of all-electric melting
While electric furnaces have lower capital costs, they have shorter life-times (2-7 years compared to 10-20 years for conventional furnaces) and higher energy costs. The economic viability of electric furnaces is closely related to the cost of electricity compared with gas. Higher thermal and energy efficiencies can offset this cost for smaller furnaces, but this might not be the case for larger furnaces.
The low environmental impact is only maintained if the furnace can receive power from renewable energy sources and requires a power grid that is reliable and stable.
There are also operational considerations. For example, the maintenance of electrodes to limit higher resistance caused by wear. It is not possible to melt higher temperature glasses (>1500C) and there is concern of corrosion/erosion of electrode material from certain glass compositions. Further, recycled glass may be an issue that requires new handling methods.
Conclusion
In most places, it is still environmentally cleaner to burn fossil fuels in a furnace than to use them to generate electricity for electric melting. However, as renewables increase their contribution to electricity production, this situation will change. It also appears that improvements in energy efficiency of fossil fuel combustion technologies have leveled off. As emissions legislation kicks in and consumers increasingly demand materials and technologies that are environmentally friendly, there may be well a swing in glass manufacture from gas to electric energy. The other advantages of electric melting, such as better thermal efficiency and energy consumption, will also count in its favor.
References
- https://www.eurotherm.com/efficient-future-for-the-glass-industry-is-all-electric
- https://www.glassmanevents.com/content-images/speakers/Andy-Reynolds-Fives.pdf
- http://www.electroglass.co.uk/articles/2010-09%20Electric%20Melting%20&%20Boosting%20for%20Glass%20Quality%20Improvement.pdf
- http://ietd.iipnetwork.org/content/electric-melting
Tuesday, 15 March 2022
Metal inclusions
Stress
Bubbles
Thin metals
Weight
Placing
Pressing
Fire in stages
Sunday, 13 March 2022
Glass Ionomers in Dental Restorations and Fillings
Glass Ionomers in Dental Restorations and Fillings
Posted Krista Grayson on Mar 27, 2019
It is widely known that eating sugary foods leads to a build-up of bacteria which can result in dental caries (tooth decay). The incidence of dental caries has fallen significantly since fluoride, which makes teeth more resilient to decay, was added to toothpaste. Nonetheless, the majority of dentist visits are still for the repair of teeth damaged by tooth decay. Indeed, in the US, 92% of adults and 21% of children have had dental caries in their permanent teeth.1
Tooth decay is caused by the acid produced by bacteria while they consume the sugars found in food and drink. This acid dissolves the protective enamel coating of teeth and then the dentine below. If the resultant cavity is not treated it can become painful, and this potentially leads to infection and even tooth loss.
The most common corrective action for tooth decay is to remove the decayed tooth tissue and fill the cavity with a filling material. There are several types of filling material currently available, including a variety of composite fillings, and the traditional silver amalgam. Composite filling materials are increasingly popular as many people prefer tooth-colored fillings that are less conspicuous. Composite dental materials can also be used for dental restorations to rebuild chipped or broken teeth. Most recently glass ionomer cements, which can be used in much the same way as composite materials, have been introduced as an additional alternative material for dental restoration.
The quality of a filling material is a key factor in determining the effectiveness of a repair. If the filling material is not durable it will be worn away during eating, and if it is prone to shrinkage bacteria will colonize the gap between the tooth and the filling giving rise to secondary caries.
The Materials used in Tooth Fillings
In the early days of modern dentistry (1800s), teeth were filled with any metal soft enough to mold into the cavity, eg, tin, silver. This advanced to dental amalgams containing a combination of metals including tin, silver, copper, and mercury as technology improved during the nineteenth century. By the end of the first quarter of the twentieth century silicate dental cements had been developed for both dental filling and the bonding of other dental restorations.2
Amalgam is still the most commonly used filling material today. Even after concerns were raised about the toxic effects of mercury, amalgam fillings continued to be used due to the inferior quality of alternatives. However, now that there are effective alternatives, which have the added aesthetic advantage of being tooth-colored, the proportion of amalgam fillings is steadily declining.
Today, there are several types of dental filling materials available, including silver amalgam, gold, porcelain, composite resins and glass ionomers. Although effective dental filling materials, gold and porcelain are rarely used due to their high costs. The other main options are compared below.
Amalgam
Amalgam is the least expensive of the dental filling materials and can be applied most quickly.2 It has the added benefit of being highly durable, lasting at least 10?15 years. There are, however, several drawbacks to the use of amalgam fillings, the most concerning of which is the potential toxicity from exposure to mercury during placement and removal of the amalgam, and also whilst in situ if an individual routinely grinds their teeth. The use of amalgam fillings also requires removal of some healthy tooth in order to create a space large enough to hold the amalgam. Lastly, the propensity of amalgam to expand and contract with changing temperature makes it more likely to crack or fracture and damage the surrounding tooth as a consequence of drinking hot and cold liquids.
Resin Composites
Dental composites can vary in formulation but all include a synthetic resin making them similar to plastics in composition. Initially, composite materials lacked the strength and durability of amalgam, but advances in their production mean that they can now be both strong and durable. Their main benefit is that they chemically bond to the tooth structure, providing further support and reducing the marginal gap that encourages bacterial colonization and increases the risk of secondary tooth decay. There is however a risk of subsequent shrinkage that can lead to gap development. Composite fillings are also aesthetically more pleasing since, unlike amalgam fillings, they blend in with the natural tooth surface. Unfortunately, composite materials are still considerably more expensive than amalgam (although still less expensive than gold or porcelain) and are more time-consuming to apply.2 Furthermore, the successful application of composite fillings is very technique sensitive and requires the area to be kept dry during placement.
Glass Ionomer Dental Cements
Glass ionomer cements (GIC) can have a variety of compositions, but the principal constituents are silica, alumina, and calcium. A source of fluoride, such as fluorite, is also commonly added to provide protection against tooth decay. Additional minerals can also be incorporated into the GIC to promote remineralisation and/or prevent acidification. The glass ionomer may be combined with resin for added strength, and to reduce the sensitivity to the presence of moisture on placement.3 GIC represent a very flexible dental restoration solution since the physical properties of GIC can be modified to meet a specific dental application by adjusting the ratios of the constituent chemicals.2
GIC, like resin composites, are tooth-colored and so have cosmetic appeal. The primary benefit of GIC is their chemical adhesion to enamel and dentin, which improves the strength of the restoration and eliminates the need for a bonding agent during placement.2,4 The bond strength of this adhesion is typically increased by addition of polycarboxylic acid. GIC have been reported to exhibit a contact-free area wear that is five times higher than that of amalgam and three times higher than for resin composite materials.2 Furthermore, in contrast to other restoration materials that can suddenly fail due to mechanical fatigue, GIC become stronger over time as water is absorbed and are thus less prone to failure.2
Most recently, GIC has been created using bioactive glass.5 Resin-modified GIC containing bioactive glass has been shown to result in a thick uniform layer of mineralization on the restoration-dentin interface,6 improve the mechanical properties of a filling,7 and reduced the incidence of secondary tooth decay at restoration margins.8
Conclusion
Despite silver amalgam being the mainstay dental filling material for many decades, there has been a desire to reduce its use due to toxicity concerns. Now that the alternative products available can provide comparable efficacy, the proportion of dental caries being corrected with amalgam fillings is declining. Advances in the formulations of composite and glass ionomer dental materials have given them the required strength and durability to make them effective products for tooth restoration. Although fillings with these newer materials are more expensive and take longer to place, they are often the preferred choice due to their improved aesthetics and low risk of toxicity.
Glass ionomer cements have the added benefits of flexibility in their physical characteristics, strong adhesion to the tooth surface and lower failure rate. The properties of both composite and glass ionomer dental materials can be improved by the inclusion of bioactive glass.
Mo-Sci produces a range of high quality glass and bioactive glass powders suitable for use as a dental filling materials and for the fixation or coating of dental implants.9 The precise composition of their glass products can be tailored to suit a specific application. Contact us for for more information.
References & Further Reading
- National Institutes of Health. NIDCR Data & Statistics. Dental Caries (Tooth Decay) in Adults (Age 20 to 64). Available at: https://www.nidcr.nih.gov/DataStatistics/FindDataByTopic/DentalCaries/DentalCariesAdults20to64.htm
- Lohbauer U. Dental Glass Ionomer Cements as Permanent Filling Materials? — Properties, Limitations Future Trends. Materials 2010, 3(1), 76-96; doi:10.3390/ma3010076
- Gao W, et al. Demineralization and remineraliza-tion of dentine caries, and the role of glass ionomer cements. Int Dent J. 2000;50(1):51–56.
- Benelli EM, et al. In situ anticariogenic potential of glass ionomer cement. Caries Res. 1993;27(4):280–284.
- Matsuya S, et al. Structure of bioactive glass and its application to glass ionomer cement. Dent Mater J. 1999 Jun;18(2):155–166.
- Prabhakar AR, et al Comparative Evaluation of the Remineralizing Effects and Surface Micro hardness of Glass Ionomer Cements Containing Bioactive Glass (S53P4):An in vitro Study. Int J Clin Pediatr Dent. 2010 May-Aug;3(2):69-77. doi: 10.5005/jp-journals-10005-1057. Available at https://www.ncbi.nlm.nih.gov/pubmed/27507915.
- Chatzistavrou X, et al. Fabrication and characterization of bioactive and antibacterial composites for dental applications. Acta Biomater. 2014;10:3723–3732. Available at https://www.ncbi.nlm.nih.gov/pubmed/24050766
- Khvostenko D, et al. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dental materials 2016;32(1):73–81. Available at http://www.demajournal.com/article/S0109-5641(15)00437-6/pdf
- Mo Sci Corporation website. http://www.mo-sci.com/en/products
Sunday, 6 March 2022
Reducing Implant-Related Infection with Bioactive Glass
Reducing Implant-Related Infection with Bioactive Glass
Posted Krista Grayson on Feb 13, 2019

Disease, trauma or serious infection may result in extensive bone damage or bone loss which exceeds the body’s capacity to repair itself. In such cases, implants are needed to promote satisfactory healing. These may take the form of screws, plates, or rods to immobilize broken bones in the correct alignment, reinforce weak bones or correct skeletal deformities. Similarly, diseased joints can be replaced with prosthetic joints to restore normal, pain-free movement.
Despite ongoing medical advances and improvements in materials and procedures, there remains a substantial risk of implanted devices becoming infected. In addition to microbes being introduced into the body during surgery, there is the risk of bacteria transported in the blood from other parts of the body colonizing the surface of an implant. It has been estimated that as many as 2.5% of primary hip and knee replacements and to 10% of joint revision surgeries are complicated by infection.1 Infected implanted devices represent a significant clinical challenge. Typically, despite lengthy antibiotic treatments, it is often necessary for the infected implant to be surgically removed. This not only increases patient morbidity and dissatisfaction, but is also associated with substantial cost.2
Antibiotics continue to be the mainstay strategy for both the prevention and treatment of implant infections. However, the power of antibiotics in the fight against infection is diminishing as many strains of potent bacteria are developing resistance to even the strongest antibiotics. Consequently, the risk of implanted devices becoming infected is on the rise and researchers are investigating novel ways to reduce such infections.
One strategy is based on the discovery that the majority of bacteria live in surface-bound microbial communities, rather than as free-swimming entities. On binding to the surface, bacteria secrete adhesion proteins that provide an irreversible attachment.3 Such bacterial biofilms account for over 80% of clinical microbial infections.2 It was therefore proposed that making the surface of implants unsuitable for bacterial colonization would dramatically lower infection rates. This can be achieved by coating the implant with bioactive glass.
The Antimicrobial Properties of Bioactive Glass
Bioactive glass is a type of glass made from high-purity chemicals, such as silica, calcium, and boron, which induce specific biological activity.4 Bioactive glass, by virtue of its high strength, low weight and biocompatibility, has been widely used in a range of biomedical applications, including tissue engineering, bone grafting, dental reconstruction and wound healing.5
Such clinical experience has shown that borate bioactive glass possesses antimicrobial properties against a wide range of bacteria, including MRSA and E-coli.6,7 The antimicrobial efficacy is achieved though an increase in pH of the surrounding body fluids (which is stressful for bacteria) and because any bacteria that do approach are unable to adhere to bioactive glass and so cannot create microfilms on its surface.8,9 In vitro studies have confirmed that bioactive glass has strong anti-staphylococcal and anti-streptococcal activity.10,11
Since the antimicrobial action of bioactive glass arises from it creating an environment that is hostile to bacteria rather than requiring direct contact with the invading microbe in order to kill it, it is effective across a wide range of bacteria. Furthermore, the bacteria cannot adapt to such effects, and so no bacteria have been found to develop resistance to the antimicrobial effects of bioactive glass.9
Coating Implants with Bioactive Glass
Initial technical challenges have been overcome and a range of titanium implants have been successfully coated with bioactive glass.12 Clinical use of implants coated with bioactive glass has given promising results in both orthopedic and dental applications. There was no evidence of the coated implants causing any adverse effects or inflammatory response in the surrounding tissue. Furthermore, the implants coated with bioactive glass were found to accelerate cell attachment and mineralization of the extracellular matrix, promoting more rapid bone growth. In addition, the proportion of bone-to-implant contact was significantly greater for implants coated with bioactive glass compared with traditional implants.13-15
Enhancing the Antimicrobial Efficacy of Bioactive Glass
Bioactive glass has good antimicrobial action, being effective against a broad spectrum of aerobic and anaerobic bacteria.9 However, the antimicrobial effects can be further enhanced to increase the range of antimicrobial activity, by the addition of ions, such as boron, copper, silver, yttrium, and iodine, or organic nanoparticles.16,17 The chosen antimicrobial agent is incorporated into the bioactive glass during its production and released once the bioactive glass is in an aqueous solution, creating an environment inhospitable for microbial life. The bioactive glass can thus be used as a delivery system for antimicrobials.18
The advantage of ions and nanoparticles over antibiotics is that their efficacy depends solely on contact with the bacterial cell wall; they do not need to enter the cell. Consequently, their lethal effect is delivered irrespective of the specific genetics of the target bacteria and is unaffected by the resistance mechanisms used by bacteria to evade antibiotics.
Conclusion
Infection of medical implants is an increasingly serious clinical and socioeconomic burden. Furthermore, the situation is likely to worsen with the increasing prevalence of bacteria with multi-drug resistance.
Bioactive glass has inherent antimicrobial activity and does not elicit a toxic response to surrounding tissues. Consequently, coating implants with bioactive glass represents an attractive option for reducing the risk of infection. The antimicrobial properties of bioactive glass can be further enhanced by loading it with antimicrobial agents, such as ions or antibacterial nanoparticles. Such a strategy would reduce the need for prophylactic antibiotic use, whereby protecting against the development of further strains of antibiotic-resistant bacteria.
Since bacteria cannot adapt to the hostile environment created by bioactive glass or its biofilm-resistant surface, they are unlikely to develop resistance to the antimicrobial action of bioactive glass.
In addition, the coating of implants with bioactive glass has been shown to speed up the fusion of the implant with bone, accelerating a patient’s recovery.
The use of bioactive glass, either alone or doped with antimicrobial agents, as a coating for orthopedic and dental implants is thus likely to improve the success rate and enhance patient outcomes across a range of reparative and restorative surgeries by promoting rapid healing and minimizing the occurrence of infection.
Mo-Sci produces high quality bioactive glass powders, the precise composition of which can be tailored to meet specific requirements. They produce bioactive glass suitable for coating orthopedic and dental implants.
References & Further Reading
- Lentino JR. Prosthetic joint infections: Bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157–61. doi: 10.1086/374554.
- Hall-Stoodley L, et al. Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews Microbiology2004;2(2): 95–108.
- Davey ME and O’Toole GA. Microbial biofilms: from ecology to molecular genetics. Microbiology and Molecular Biology Reviews2000;64(4):847–867.
- Brauer DS. Bioactive Glasses—Structure and Properties. Angew Chem Int Ed 2015;54: 4160–4181.
- Rahaman MN, et al. Bioactive glass in tissue engineering. Acta Biomaterialia 2011;7:2355?2373.
- Ottomeyer M, et al. Broad-Spectrum Antibacterial Characteristics of Four Novel Borate-Based Bioactive Glasses. Advances in Microbiology 2016;6:776?787.
- Khvostenko D, et al. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dental materials 2016;32(1):73–81. Available at http://www.demajournal.com/article/S0109-5641(15)00437-6/pdf
- Zhang D, et al. Factors Controlling Antibacterial Properties of Bioactive Glasses. Key Engineering Materials 2007;330-332:173?176.
- Drago L, et al. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review Materials 2018;11:326?337.
- Misra SK, et al. Poly(3-hydroxybutyrate) multifunctional composite scaffolds for tissue engineering applications. Biomaterials 2010;31:2806–2815.
- Rivadeneira J, et al. In vitro antistaphylococcal effects of a novel 45S5 bioglass/agar-gelatin biocomposite films. J Appl Microbiol 2013;115,604–612.
- Lopez-Esteban S, et al. Bioactive glass coatings for orthopedic metallic implants. Journal of the European Ceramic Society 2003;23:2921–2930.
- Mehdikhani-Nahrkhalaji M, et al. Biodegradable nanocomposite coatings accelerate bone healing: In vivo evaluation. Dent Res J (Isfahan). 2015;12(1):89?99.
- Chen Q, et al.Cellulose Nanocrystals–Bioactive Glass Hybrid Coating as Bone Substitutes by Electrophoretic Co-deposition: In Situ Control of Mineralization of Bioactive Glass and Enhancement of Osteoblastic Performance. ACS Appl Mater Interfaces. 2015 Nov 11;7(44):24715?25.
- van Oirschot BA, et al. Comparison of different surface modifications for titanium implants installed into the goat iliac crest. Clin Oral Implants Res. 2016;27(2):e57?67.
- Kaur G, et al D. Review and the state of the art: Sol–gel and melt quenched bioactive glasses for tissue engineering. J Biomed Mater Res B Appl Biomater 2016;104, 1248–1275.
- Karwowska E. Antibacterial potential of nanocomposite-based materials – a short review. Nanotechnology Reviews 2016;6(2):243?254.
- Rivadeneira J and Gorustovich J. Bioactive glasses as delivery systems for antimicrobial agents. Journal of Applied Microbiology 2016;122, 1424–1437.
- Mo Sci Corporation website. http://www.mo-sci.com/en/products

