Releasing glass from steel
Releasing glass from ceramic
 |
| A ceramic draping mould from which it may be difficult to remove the glass. |
 |
| A ceramic draping mould from which it may be difficult to remove the glass. |
Posted Krista Grayson on Jan 5, 2018
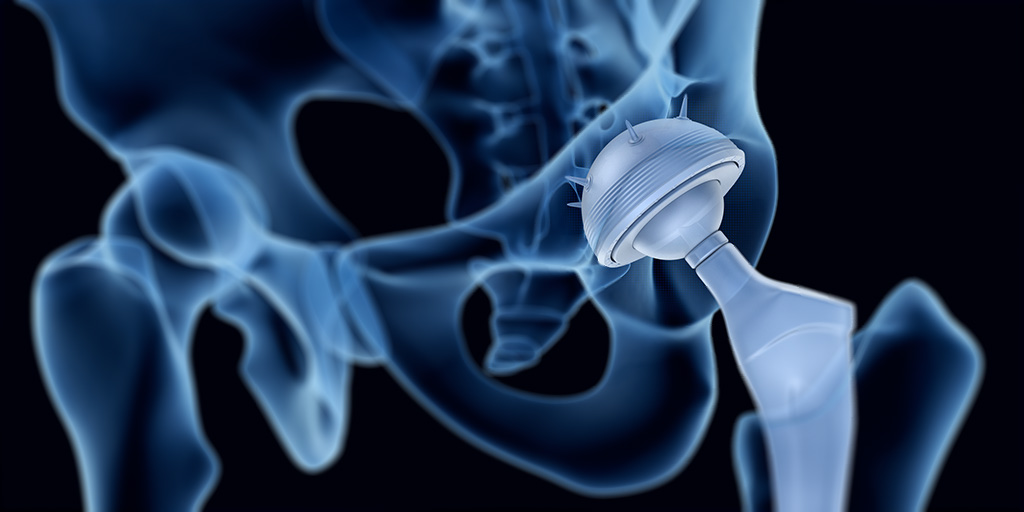
Bioactive glass induces specific biological activity when implanted in the body that causes the glass to become covered with a substance similar to hydroxyapatite. The formation of this layer allows bioactive glass to bond firmly with both hard and soft tissues.
This behavior has instigated extensive research into the use of bioactive glass to facilitate the repair of damaged bone. Furthermore, these bioactive glasses are biocompatible, and so do not elicit immune responses that can lead to rejection of foreign materials introduced into the human body.
Although brittle, bioactive glass offers a strong, yet lightweight, biodegradable framework to support healing. Furthermore, new borate and borosilicate bioactive glasses have been shown to enhance bone regeneration. In addition, the composition of bioactive glass can be adjusted to determine how long it persists in the body before it degrades so it provides support as long as is needed.1
Considerable success has been achieved using bioactive glass in the repair of bone, soft tissue and cartilage.1 Bioactive glass has also been shown to be beneficial in periodontal reconstruction2 and to promote the healing of ulcers in patients at risk of leg amputation.3
The efficacy of bioactive glass in enhancing the healing of both hard and soft tissues led to investigation into their application in reconstructive surgery requiring the use of permanent implants. This article explores how bioactive glass can help encourage the integration of implants.
The human body has an amazing capacity to heal itself. However, in the case of multiple or complex fractures or serious infection or disease there can be too much of the original bone missing for it to heal satisfactorily. In such cases a bone graft or scaffold is required to support regeneration or bone fusion. Similarly implanted devices, such as plates, or screws and joint replacements, may be needed to reinforce or replace weak or damaged bones and joints. Similarly, implants may be required in dentistry to facilitate artificial replacement of a tooth root. These are usually in the form of a metallic screw positioned in the jaw bone that can support one or more false teeth.
Bone taken from another part of the patient’s body, an autograft, is the preferred implant for bone repair since it will not be at risk of rejection. However, this can be hampered by availability or suitability and incurs additional morbidity for the patient at the site from which the graft is harvested.
Titanium alloy implants have good biological compatibility but, in order for the implant to provide a strong structural support, it must be integrated into the bone (osseointegration) and it can take several months for the bone to grow in and/or around the implant. The implant must also be mechanically and morphologically compatible so it maintains good contact with the recipient bone to promote bony cell growth. In addition, there is the risk of metal implants being corroded by body fluids and releasing potentially toxic products.
Consequently, there has been much research into coatings for prosthetic metallic implants.4
Bioactive glass is one such coating material that is currently the focus of much research. Once in the body, an amorphous calcium phosphate layer forms on the surface of bioactive glass. Within hours, this layer incorporates blood proteins and collagen and crystallizes into hydroxycarbonate apatite. This layer is now very similar to natural bone mineral, and so bonds readily to the recipient tissues/bone. Bioactive glass is therefore a prime candidate for coating implants that need to become integrated into bone.
Including bioactive glass in polymeric scaffolding materials has been shown to accelerate the formation of a strong bond between the scaffold and tissue and promote healing.5 It followed that similar technologies may facilitate the osseointegration of the implants.
Numerous technical challenges have been overcome and titanium implants have been successfully coated with bioactive glass4 and evaluations of bioactive glass-coated implants have had promising results.6,7,8 Bioactive glass coatings on both orthopaedic and dental implants were shown not induce any adverse effects or inflammatory response in the surrounding tissue6. Furthermore, bioactive glass coatings accelerated cell attachment, spreading, proliferation, differentiation, and mineralization of the extracellular matrix and promoted rapid bone growth.6,7 In addition, the proportion of bone-to-implant contact were significantly greater for implants coated with bioactive glass.8
Bioactive glass can be obtained in a range of sizes and compositions9 suited to a range of applications. Indeed, bioactive glass can be custom made to specifications that precisely match a specific need, in terms of strength, degradation rate etc.
Implants are commonly needed in orthopaedic surgery to facilitate the repair of damaged or missing bone and in dental reconstructions. Although implants have been used with great success, procedures may be limited by rejection issues and toxicity concerns. Furthermore, it can take several months for an implant to become integrated into the recipient bone and this increases the risk of failure and prolongs recovery times.
The biocompatibility and strength of bioactive glass along with its ability to promote tissue regeneration has made it an invaluable tool in tissue engineering. With recent technological advances, it is now possible to coat metal implants with bioactive glass. Implants coated in this way have demonstrated great advantages in terms of both patient safety and recovery. The bioactive glass coating protects the metal implant from corrosion by bodily fluids thereby minimizing the risk of potentially toxic products entering the body. It also promotes new bone growth so the implant becomes secured in the bone more rapidly.
Bioactive glass coatings on implants can be used to encourage implant fixation, improve healing rates and maximize implant effectiveness.
Mo-Sci produce high quality bioactive glass in a form suitable for coating implants and can tailor its composition to meet specific requirements.
Posted Krista Grayson on Dec 4, 2017
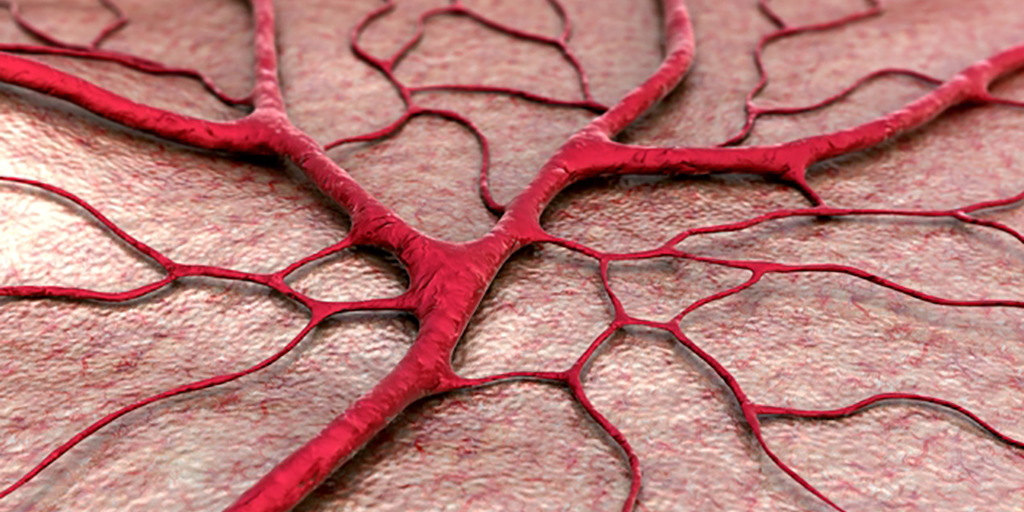
Developments in tissue engineering over recent years have made possible the restoration of serious trauma.1 Using temporary scaffolds of biologically compatible substitutes, such as bioengineered tissue, damaged, injured or missing body tissues can be replaced.
With significant advances in the available scaffolds for use in tissue engineering, the provision of an adequate blood vessel system — vascularization — has become the key limitation to the regeneration of tissues after trauma.2
Several approaches have been used to achieve the necessary vascularization to supply sufficient blood to bioengineered tissue. These include loading the scaffold with angiogenic growth factors, such as vascular endothelial cell growth factor (VEGF), or endothelial cells and even prevascularization of the tissue to be implanted.2
Bioactive glasses have been shown to provide effective scaffolds for soft tissue engineering.1 They are biocompatible, lightweight and strong, and can be produced to degrade at a rate that matches the growth of new tissue.1 More recently, it has become apparent that bioactive glass also promotes angiogenesis that is important for supporting new tissue growth.
The human body has a remarkable ability to heal itself after undergoing trauma. However, such healing is a complex biological process requiring many different cell types to complete the necessary steps at the right time.3 If large amounts of tissue have been lost, tissue engineering is used to provide a temporary biomaterial scaffold to provide the necessary support or shape while the new tissue grows.
Initially, damaged blood vessels must constrict to stem blood loss but then they are required to regenerate to provide nutrients to the new tissue created to restore the damage. Vascular regeneration is thus an important step in the healing process. If there is not an adequate vascular system, the nutrients required for growth cannot be supplied.
Bioactive glass, by virtue of its biocompatibility, strength and range of achievable properties, is widely used to provide support in tissue engineering and been used with much success in the repair of bone, soft tissue and cartilage repair.1 Once implanted in the body, reactions occur on the surface of bioactive glass that facilitate bonding with existing tissue. Furthermore, bioactive glass can release ions, such as calcium that is important for regeneration of skin and bone, that are needed to support regeneration and promote rapid bone formation.4
More recently it has also become apparent that bioactive glass can promote vascularization without the need for adding growth factors to the scaffold.5-7
Bioactive glass is a valuable tool in tissue engineering. Although originally used to facilitate bone repair, it has also provided tremendous benefit when included as a component of bioscaffold materials used in soft tissue repair. Bioactive glass has been shown to speed up healing and has the added benefit that its rate of resorption can be tailored to meet a particular repair need.1
A novel form of borate bioactive glass has been successfully used in wound healing.8 A fibrous network of calcium-rich glass fibers forms a scaffold to promote skin regeneration. When this bioactive glass was used in patients with diabetic ulcers who were at risk of limb amputation, the skin was fully repaired in almost two thirds of cases after a few months with little if any scarring.8
More recently, it has been shown that bioactive glass actively enhances tissue regeneration by stimulating the secretion of angiogenic growth factors that promote the proliferation of microvascular endothelial cells and enhance revascularization.5,6 Thus, bioactive glass is able to augment a critical process in tissue regeneration.7 This allows more rapid tissue repair without the need for adding recombinant inductive growth factors.
Bioactive glass is established as a key tool in a range of tissue engineering applications. It promotes the repair of bone and soft tissue whilst providing structural support. Furthermore, the bioactive glass can be designed to last just as long as is needed for the new tissue to gain the necessary volume and strength for the repair to be completed.
In addition, it has been shown that bioactive glass also increases the levels of angiogenic growth factor, which accelerate vascular regeneration. Vascularization is a key process in tissue repair since blood vessels are required to provide the nutrient for new tissue to develop and grow. Previously, recombinant growth factors were added to tissue engineering scaffolds to promote the creation of new blood vessels. This extra process can now be obviated by including bioactive glass as a component of the scaffold material.
Proangiogenic potential is thus another desirable quality that can be added to the properties of bioactive glass. This further supports the use of bioactive glass in temporary healing scaffolds during tissue engineering procedures.
Mo-Sci produces medical implant grade bioactive glass in a range of formats suitable for use in a wide variety of tissue engineering scaffolds and can tailor its composition to meet specific requirements.9
Posted Krista Grayson on Nov 17, 2017
Wound healing is a complex and dynamic biological process that requires different types of cells to complete the critical steps at the appropriate time promptly1. When blood vessels are damaged, they constrict and dilate intermittently. The vessels must constrict to stop blood loss and then dilate again to allow the immune system deal with the invading microorganisms such as bacteria. A scaffold is then formed by a network of fibrin, allowing a proliferation of new cells to again populate the site of injury.
Wound healing is usually taken for granted; however, there are many factors that can affect this complex process, such as medications, infection, and lack of oxygen. Age and diabetes are considered the top risk factors for impaired or delayed wound healing.
Diabetes and aging can result in blood and other body fluids that accumulate in the lower limbs and feet owing to damaged valves or stretched veins, and therefore prevent these fluids from being pumped back to the heart. When more and more fluid accumulates, it increases the pressure which, in turn, causes the accumulated fluids to seep through the skin. This triggers a venous stasis ulcer.
The fluids that seep from the wound include enzymes that clean a wound during the early stages of the healing process. However, when these fluids exude continually, they impede the next step of the healing process and make the wound even worse. In many cases, limb amputation1 provides the only solution for treating repeated infection and long-term non-healing wounds.
About 1% of people in industrialized countries suffer from leg ulcers, which are mainly attributed to poor flow of blood from the legs to the heart. In spite of treatment, some ulcers do not heal even after months or years. As a result, intense research has been made on specialized wound-care treatments capable of promoting the healing of leg ulcers.
Fluid accumulation can be prevented through compression of the lower leg. This can stop the fluid from seeping through a venous stasis ulcer and allow the wound healing process to carry on2. Likewise, fluid accumulation in the wound can be prevented by applying a vacuum to the ulcer, and thus promote the healing process3.
While these approaches can be effective, they are very expensive and have to be continued for an indefinite period of time, and most importantly, they are inconvenient for patients. Further, there is no guarantee that wound healing will be achieved.
One innovative wound care approach is adding glass fibers to develop a scaffold that promotes the formation of new tissue and thus helps in wound closure4. This scaffold is similar to the natural scaffold provided by fibrin during the wound healing process.
Over recent years, breakthrough developments in tissue engineering have made it possible to reverse the damage caused by disease or trauma5. A temporary biomaterial scaffold that provides the required shape or support while the new tissue grows represents an important aspect of all tissue engineering. Bioactive glass, in terms of its strength, biocompatibility, and range of attainable properties, is broadly used to extend support in tissue engineering.
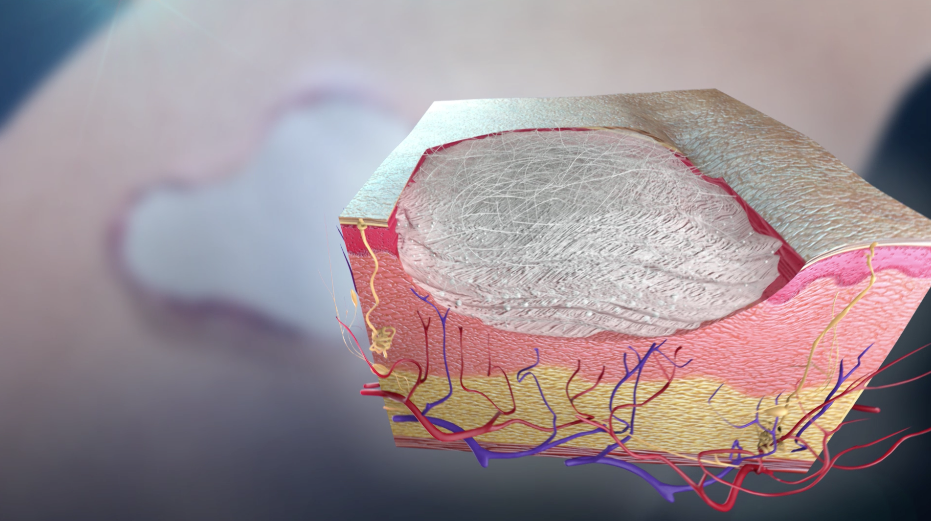
So far, silica-based bioactive glasses have been traditionally used to facilitate periodontal reconstruction or bone repair, but now many new borate-based bioactive glasses are used as scaffolds for soft tissue engineering5. Borate bioactive glass scaffolds provide the required support and have also been shown to promote angiogenesis, a key process that promotes the growth of new tissues5.
Recently, a new form of borate bioactive glass has been developed by Mo-Sci Corporation (Rolla, Missouri, USA) for wound healing (DermaFuse™/Mirragen™)4,6. Tiny cotton-like fibers are drawn out from the bioactive glass. A scaffold is produced by the fibrous network, similar to the natural fibrin scaffold formed by the body, to encourage wound healing. There is high calcium content in the glass because this mineral is essential to promote skin regeneration. It has been shown that the fibrous borate bioactive glass is effective and help heals long-term venous stasis ulcers. It is being hoped that this glass will also be equally effective for treating burns and other extensive wounds.
At Phelps County Regional Medical Center, USA, a clinical trial was performed that demonstrated that DermaFuse (now known as Mirragen™) was highly effective in diabetic ulcer patients at an increased risk of limb amputation. Among the 13 participants, some had wounds that had not healed for over a year. When the wound was packed for a few months with the fibrous borate glass, the skin was completely healed in eight patients while showed considerable improvement in the other four participants. In fact, the healed skin had little to no scarring.
Earlier this year, this new wound healing material obtained FDA marketing approval and is also received approval for use in the veterinary field under the brand name Redi-Heal™.
In tissue engineering, bioactive glass serves as an important tool as it is biocompatible and also its properties can be customized to meet a specific requirement by modifying the glass’ structure and composition. For many years, bioactive glass has been used to provide a scaffold for periodontal reconstruction and bone repair, and more recently it has been widely studied in soft tissue repair.
In addition, a fibrous bioactive glass product was approved for use in wound repair earlier this year. The effective treatment of non-healing venous stasis ulcers shows that more serious skin damage such as burns may also be effectively treated.
Posted by Krista Grayson on Sep 26, 2017
Researchers are now combining advanced materials like bioactive glasses and 3D printing techniques to create custom scaffolds and implants that dissolve in the body and are replaced with new tissues.
3D printing, also known as additive manufacturing, is already widely utilized in the medical industry. Hearing aids are routinely 3D printed, and there have been numerous reports of 3D printers producing patient-specific implants made from plastic or metal.1-4
Researchers are now combining advanced materials like bioactive glasses and 3D printing techniques to create custom scaffolds and implants that dissolve in the body and are replaced with new tissues.
Bioactive glasses provide the ideal synthetic materials for regenerative procedures such as bone grafting (Figure 1). Bioactive glasses are phosphosilicate materials that contain sodium and calcium. In the body, the glasses bind strongly to tissues and provide surfaces for new cell and tissue growth.
The glass eventually dissolves and releases calcium into the blood, which reacts to make hydroxylapatite, a hard and rigid mineral that is a key component of bone. In this way, bioactive glasses can aid the regeneration of bone. The composition of bioactive glasses can be tailored to give the glasses therapeutic, antimicrobial, and cell recruiting effects. Furthermore, bioactive glasses can be combined with other materials to create composites with a variety of properties, resulting in a wide range of medical applications.8,9
Steve Jung, CTO at Mo-Sci Corporation, described the advantageous properties of bioactive glass “Since its inorganic, it’s essentially a limitless supply; you can always make more, whereas bone or other types of materials used in medical applications you need cadaver or patient supplied bone, and sometimes there’s not enough”.
As bioactive glass grafts are man-made, they are also relatively inexpensive and provide no potential for disease transmission.8
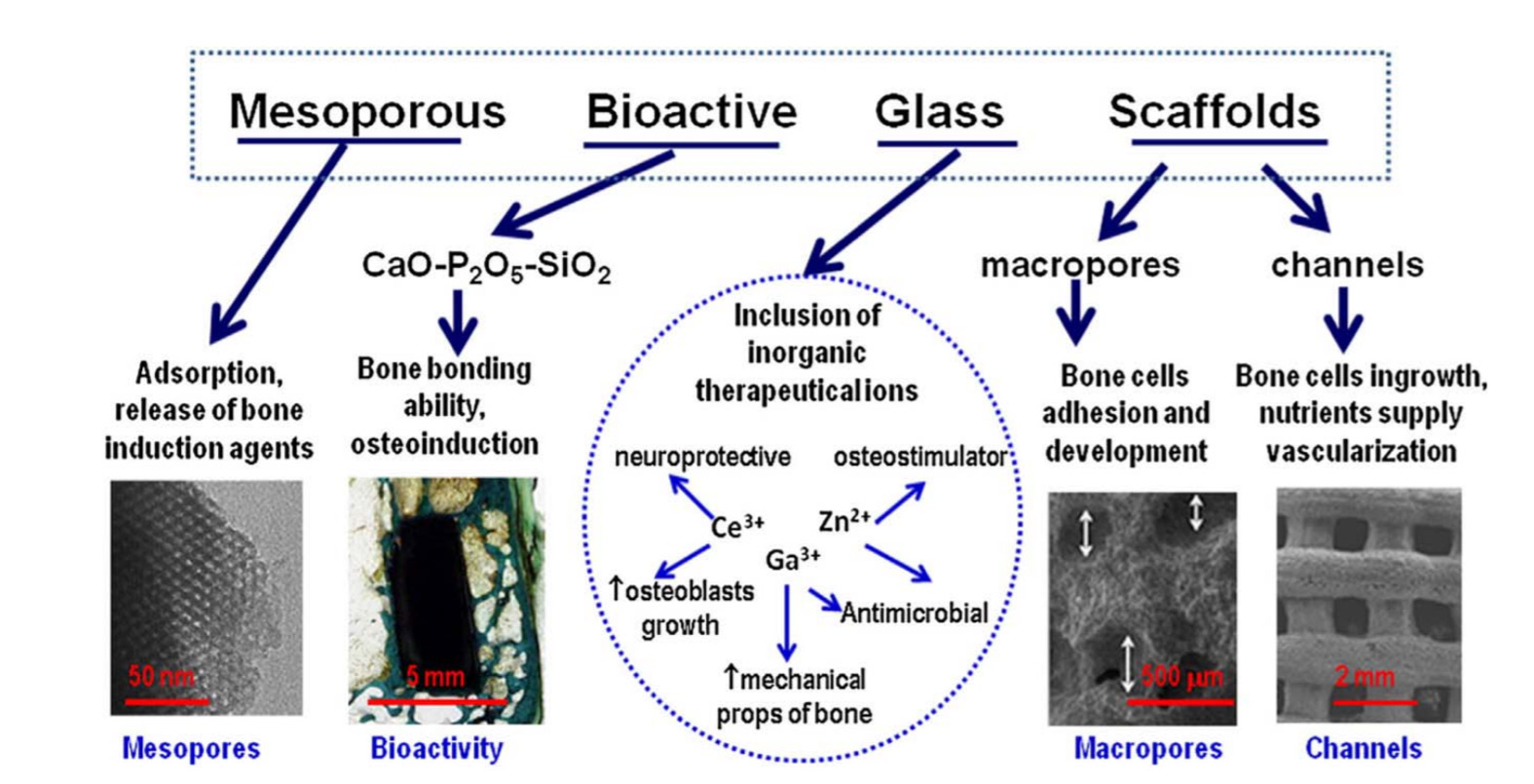
Figure 1. Features that make bioactive glasses optimum candidates for bone tissue engineering.10
The use of particles and putties of bioactive glass in clinical practice to support bone regeneration is widespread and has been used in more than a million patients.9,11 Bioactive glasses can also be used to make scaffolds to support tissue regeneration in larger areas. Bioactive glass scaffolds can be produced using foaming methods, resulting in scaffolds with pore structures that mimic the structure of bone (Figure 2).
However, it can be difficult to control the pore architectures of scaffolds produced by foaming, and the resulting scaffolds are relatively brittle. Surgeons often require scaffolds for bone grafts that have precise pore architectures and can be load bearing. 3D printing can produce bioactive glass structures with finely controlled pore structures (Figure 2) and increased mechanical strength.9,12
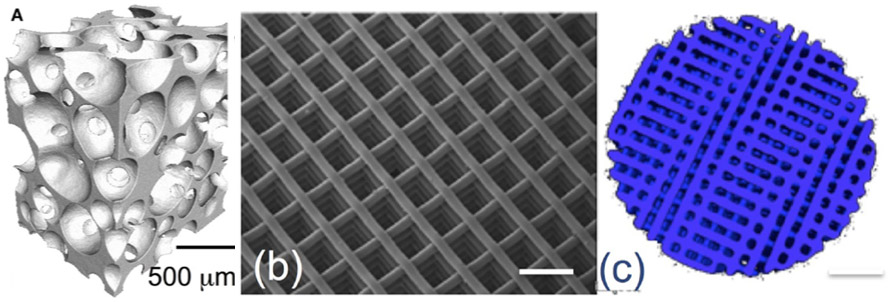
Figure 2. X-ray microtomography images of a bioactive glass scaffold produced using sol-gel foaming (a) and 3D printing (b,c).12
3D printing is a process that produces 3D structures from a digital model by laying down many layers of a material. Typically, 3D printing uses polymers or metals to produce structures, but researchers are now able to 3D print bioactive glass materials and composites. This enables bioactive glass scaffolds to be precisely designed in terms of their pore architecture and the final shape of the scaffold.13-15 3D printed structures made from bioactive glass could be used for novel solutions in medical implants, dental implants, surgery, and tissue scaffolding. The use of 3D printing means that a patient can be scanned, and then a unique implant or scaffold can be designed and printed with the correct size and properties for them.9,16
Although the use of 3D printed bioactive glasses is not yet widespread, there have been numerous investigations into their use in both animal models and human patients requiring unique, custom solutions (Figure 3). Research into the potential of 3D printed bioactive glasses and composites is ongoing, and the process of 3D printing bioactive glass structures is still being optimized, particularly with regards to optimizing the porosity and mechanical strength of the resulting scaffolds, and selecting the most appropriate binder materials and post processing techniques.16-18
There is also ongoing research into incorporating live cells, growth factors, and drugs into bioactive glass scaffolds using 3D printing.16,19
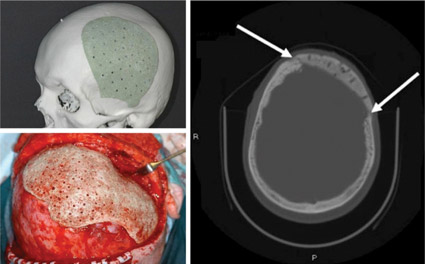
Figure 3. Photos of a tailor-made bioactive glass composite implant before operation (above, left), and during surgery (below, left). A CT scan image 2 years after reconstruction (right). New bone formation between the implant and surrounding bone is seen (white arrows).20
3D printing bioactive glass scaffolds can produce precisely designed, custom scaffolds for bone grafting. However, the process of printing bioactive glasses is still under optimization.
Mo-Sci offers a wide variety of bioactive glasses for both research and medical applications, with custom compositions available upon request.21
This is a note from Christopher Jeffree on a piece of research he did on the effects of three chemicals to remove kiln wash and investment residue from glass. These are the common vinegar soak, my preferred citric acid soak and a tri-sodium citrate soak.
This latter is a neutralised citric acid. It is widely used in the food, and engineering industries. It is an anti-oxidant. It is used to remove limescale also. Clearly it is an all around useful chemical. It is edible, widely available, and cheap.
Christopher informs me that "One interesting application for it is for retarding the setting of gypsum plaster, so it is sold by plasterers and building merchants." It is also available through Amazon, Ebay and sellers of food making supplies. Typically, it is sold as tri-sodium citrate dihydrate.
Without more introduction, here is Christopher's research and conclusions.
--- --- --- --- --- --- --- --- --- ---
Which etches glass more – 6% vinegar or 6% citric acid? To
cut a long story short, a quick experiment shows that it depends on the glass.
·
Both acids etch opal glasses, especially some reds,
oranges and yellows, when soaked for 48h, but citric acid etches the same
colours more in the same time.
·
Most transparent colours and clears are very
resistant to etching, even when exposed for much longer times.
·
The neutralized form of citric acid, tri-sodium
citrate, is just as effective as citric acid for cleaning glass of mould
material and kiln wash but does not etch either transparents or opals during
extended soaks of several days.
·
Bottom line:
to avoid glass etching, long soaks should be carried out in trisodium
citrate, not in vinegar or citric acid
©Chris Jeffree, December 2021