The Identification of stress is important in investigating the causes of stress. We have well established clues to help us with our glass selection and alteration of our firing schedules. We can get more information about why the cold glass has broken from the scientific literature. The manufacturers of float glass and the installers of large panes investigate thoroughly the causes of breaks in glass that has been installed.
One article - Breaking It Down, Why Did the Glass Break? by Timothy Bellovary from Vitro Architectural Glass - looks at mechanical and thermal stress and distinguishing between the two. This post is quoted extracts from that article. [Text in square brackets are interpolations of mine]. Note that all the illustrations are from the article and are copyrighted.
Source: https://vcn.vitroglazings.com/technical-forumdiagnosing-glass-breakage
Identifying the break origin can provide hints about the following:
·
Mode of
glass failure—Was it mechanical or thermally induced stress?
·
The
stress or tension level at which the breakage occurred.
·
Other
contributing factors—were there digs (deep, short scratches) resulting from
glass-to-glass or glass-to-metal contact? Did a projectile hit the glass? Is
there edge or surface damage?
To find the origin
of a break, the first step is to assess its direction by inspecting the
fracture lines… in the glass. These rib-shaped marks, distinguished by a
wave-like pattern, begin at the break origin and radiate along break branches,
and almost always project into the concave face of these lines.
Figure 1
Diagram of Fracture Line Direction
It’s often helpful to make a basic diagram (see Figure 1) of the
fracture lines. … The origin of the break can be determined by:
·
Drawing
arrows (indicating fracture line direction) pointing into the concave face of
break wave markings in the glass edge.
·
Tracing
point-to-tail of arrows back to the break origin.
Mechanical Stress
Low-stress tension breaks are experienced most
frequently by residential window and IGU manufacturers. The origin of the break
is typically at damaged areas of the edge or surfaces near the edge, such as
digs, scratches or chips. In many cases, breakage from damaged glass occurs
after the initial edge damage is incurred, such as during IGU fabrication,
sashing operations, transportation, job-site handling or storage, or the
installation process.
In Figure 2, the break origin is not 90 degrees to the edge
of the glass, indicating a tension break caused by bending. Low-stress,
mechanical tension breaks often occur from bending at less than 1,500 psi.
Figure 2
Low-Stress Mechanical Tension Break
High-stress tension breaks share one
characteristic with low-stress tension breaks: The break origin is not 90
degrees to the edge of the glass, suggesting a tension break caused by bending.
However, additional branching of the crack within two inches of the break
origin (see Figure 3) indicates that the stress at breakage was likely higher
than 1,500 psi.
…
Figure 3
High-Stress Mechanical Tension Break
Thermal Stress
Thermal stress
breaks often originate at the edge of the glass and form virtually 90-degree
angles to the edge and surface of the glass.
As with mechanical
stress, there are two types of thermal stress breaks: low stress and high
stress.
Low-Stress Thermal Break
Low-stress
thermal breaks are
often indicated by a single break line starting at the break origin point at or
near the glass edge and propagating two inches or more before branching into
more break lines (see Figure 4). Damaged glass edges are the most frequent
cause of low-stress thermal breakage.
High-stress
thermal breaks appear
as a single break line starting at the break origin point at or near the glass
edge and generally branching into additional breaks within two
inches [50mm] of the origin. This indicates a breakage brought on by conditions
that cause high thermal stress, such as severe outdoor shading on parts of the
glazing; heating registers located between the glass and indoor shading
devices; closed, light-colored drapes located close to the glass; or glazing in
massive concrete, stone or similar framing.
Figure 5
High-Stress Thermal Break
Analysing the Break Origin
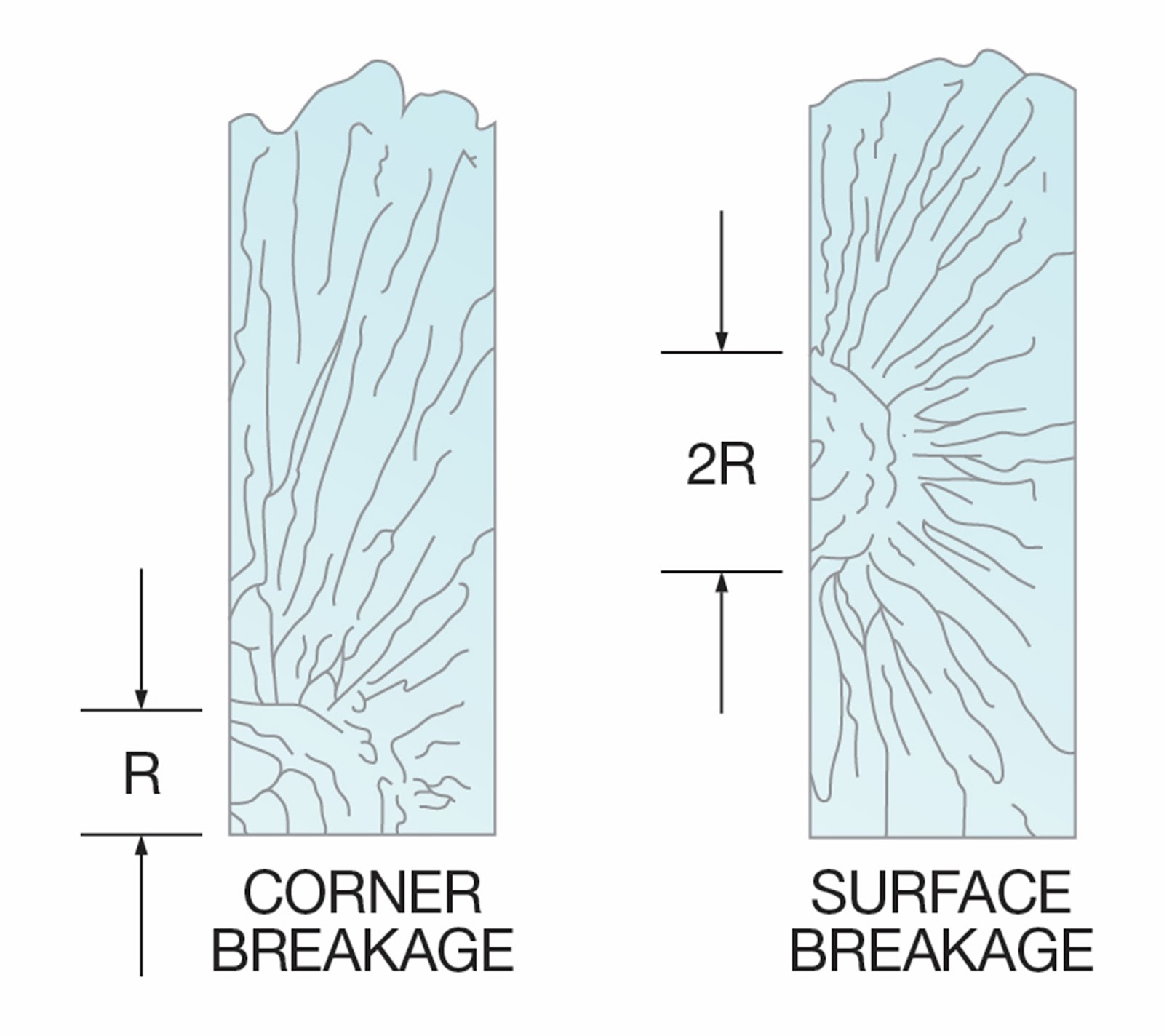
A reliable method for estimating the stress level of a
break at failure is a mirror radius measurement. Radius dimensions are
determined by crack propagation velocity characteristics.
A crack propagates itself through glass with increasing
velocity as it moves further from the point of origin. If an object has
sufficient energy to propagate a crack through the thickness of the glass, then
a “spider web” pattern will form. ….
Near the point of origin, a smooth, mirror-like appearance
on the fracture face indicates a low crack velocity. However, as velocity
increases (due to higher tension stress), the fracture face takes on a frosted
look; then, at the highest velocity, it assumes a ragged or hackled appearance.
Mirror radii appear in various forms, depending on the stress level of the
fracture.
Figure 6 shows break origins resulting from high tensile
stresses, such as bending or thermal stress breaks.
High-Stress Mirror Radii
(R = Mirror radii)
Figure 7 represents the break origins of glass fracturing
at low bending stresses. In this example, a smooth fracture face forms across
the thickness of the substrate. When the breaking stress is low, the mirror
radius is often radial and may extend deeply into the substrate.
Low-Stress Mirror Radii
(R = Mirror radii)
To identify what damaged the
glass in the first place, four factors are examined during this analysis:
·
Impact
·
Inclusions
· Thermal variance
· Pressure differentials
Impact
Identifying the nature of the breakage pattern can
determine whether a foreign object hit the glass and whether the impact was
perpendicular or parallel.
Depending on the severity of the impact, the immediate area
surrounding the break origin might be cracked, crushed or missing.
Figure 9
High-Stress Mechanical Breakage
[This pattern of break is often exhibited when the
separator fails or is insufficient to keep the glass from sticking to the
ceramic support shelf.] …
Inclusions
Any undesirable material embedded in glass is considered an
inclusion. ... [In general, kilnformers place inclusions within the glass and
know the risks of breaks].
Thermal Variance
[This article relates to float glass installations, but the
principle remains.] If the temperature difference across a [piece] of glass is
great enough, the accompanying stresses can reach levels that cause breakage. …
The combination of contact, surface damage and localized temperature gradients
can greatly increase the likelihood of breakage.
Pressure Differentials
[This section applies mainly to Insulated Glazing Units. It
points out that differences in altitude between the manufacturing and
installation sites – in combination with temperature – can cause breaks. It is
not of primary importance to most kilnforming, but something which should be considered
when installing kilnformed glass in an IGU]
Conclusion
[Occasionally] glass breaks for no obvious reason. Whether
it’s a one-off or part of a continuing pattern of incidents, glass breakage is
inconvenient, potentially dangerous and costly. … Conducting “post-mortems” on
glass breaks helps investigators identify the general reasons for each
incident, including the type of failure that caused the break, and the
potential original source of the damage. By using the techniques outlined in
this article, [kilnformers] may be able to accurately identify the likely
origin of such failures and … use that information to prevent future
occurrences.
https://vcn.vitroglazings.com/technical-forumdiagnosing-glass-breakage
[An important element in identifying breaks in kilnforming
that this article demonstrates is the difference in the angle of the break. A
90 degree angle to the surface indicates a thermal cause to the break. The more
branching of the lines of breakage, the greater the stress. The branching
breaks indicate there was significant temperature difference.
The breaks which are less than a right angle to the surface
indicate a mechanical origin of the stress. This is usually the glass breaking
at a weak point when subject to a bending stress.
If the point of origin of the stress can be identified as
demonstrated in the article, it may help in determining causes. One of these
causes might be hot or cold spots in the kiln.]