The Application
Show your best pictures
Return your
application on time
Get feedback
 |
| https askharriete.typepad.comask_harriete201212responsibilities-of-craft-show-organizers.html |
 |
| https askharriete.typepad.comask_harriete201212responsibilities-of-craft-show-organizers.html |
Posted Krista Grayson on Jul 27, 2021

Quartz, fused quartz, quartz glass, silica, fused silica… the list of terms used to describe various silica-based materials is long, confusing, and often misunderstood. In this article, we take a close look at the unique properties of quartz and fused silica (and a few related materials), and clear up the confusion surrounding these terms.
The first important thing to know about quartz and fused silica is that they both primarily consist of the same ingredient: silica, also known as silicon dioxide. Silica has the chemical formula SiO2 and is the primary constituent of most types of glass. The main form in which silica is found in nature is the mineral quartz: a hard, transparent crystalline material that makes up an appreciable fraction of the Earth’s crust. While quartz primarily consists of silica, it also contains naturally occurring impurities in various proportions depending on its geological origin.
So, silica is a specific chemical compound, silicon dioxide, with the chemical formula SiO2. On the other hand, quartz is a naturally occurring crystalline mineral, which consists primarily of silica but contains some impurities.
To fully understand the differences between different silica-based materials, we first need to review the fundamental differences between crystalline solids and amorphous solids.
The distinction comes down to how atoms are arranged inside the solids. In a crystalline solid, the constituent atoms are arranged in regular, repeating patterns known as crystal lattices. Quartz is an example of a crystalline silica-based material: silicon and oxygen atoms are arranged in a well-defined ordered structure.
However, in an amorphous solid, the atoms have no long-range order. The seemingly random arrangement of molecules in an amorphous solid resembles that of a liquid, except that they are fixed in place and don’t move around. Most materials that we think of as “glass” are amorphous solids: in fact, any material with an amorphous atomic structure can be described as “glassy”.
Whether atoms are arranged in an orderly manner or oriented randomly can profoundly influence a material’s characteristics. One of the most striking examples is the glass transition effect exhibited by amorphous solids. Outside the world of silica or other oxide-based materials, disordered “glassy” metals are often used for their unusual mechanical characteristics compared to conventional metals.1
Silica-based materials – like quartz – can be characterized both in terms of their chemical composition and whether they are crystalline or amorphous.
Now that we’ve reviewed some important fundamentals, we can define the differences between quartz, fused silica, and other silica-based materials.
As mentioned previously, quartz is the main form in which silica occurs in nature. Quartz is a crystalline solid; so, while it can resemble glass both in terms of its appearance and its chemical makeup, it has very distinct properties from glass.
Industrial applications of quartz (that is, the crystalline mineral) are limited, but include quartz crystal oscillators in electronic systems – most familiarly in wristwatches.
Perhaps confusingly, “synthetic quartz” can be manufactured for industrial quartz applications. This would perhaps be better referred to as crystalline silica, but is often referred to simply as “quartz.”
Here, the word “fused” refers to a processing step: fused silica is nominally pure silica that has been melted and cooled to form a glassy, amorphous solid. Fused silica resembles other glasses in many ways; but it does not contain any additives. Fused silica is a specialty material with a number of high-performance applications.
The terms “fused silica” and “fused quartz” are often used interchangeably. More accurately, “fused quartz” refers to an amorphous solid formed by melting naturally-occurring quartz. So, while fused silica is ostensibly pure SiO2, fused quartz contains impurities depending on the quartz that was used.
These terms are typically used in a more generic sense, and can usually be considered interchangeable. Both of these terms could refer either to fused silica or fused quartz.
While fused silica is chemically similar to quartz, its amorphous structure gives it a number of distinct and highly desirable thermal, mechanical and electrical properties.
Glasses commonly contain additives such as alkali, alkaline earth, or other oxides to lower the glass processing (melting) temperature and to improve chemical and physical properties – but fused silica is very pure. Consequently, it has higher working temperatures but offers different characteristics from other glasses.
Fused silica has a very low coefficient of thermal expansion, meaning it does not expand or contract much when heated or cooled. As a result, fused silica is highly resistant to thermal shock and can withstand very rapid heating or cooling without cracking. The thermal characteristics of fused silica make it highly valuable for high-temperature industrial components such as crucibles, trays, and boats for steelmaking and glass manufacture.2
Fused silica is transparent to a very wide spectrum of light, extending from deep ultraviolet to far-infrared. This makes it a key component in optical fibers, as well as in a range of lenses, mirrors, and other UV- or IR-transmitting optics.3,4
Fused silica is also extremely chemically inert and resistant to most acids (with the notable exception of hydrofluoric acid). This chemical inertness lends fused silica to biomedical applications, often taking the form of porous silica.
The combination of thermal stability, transparency, and strength makes fused silica a strong candidate for new and developing applications such as photolithography substrates, etched microwave circuits, and as a protective layer in semiconductor devices.
Mo-Sci develops and manufactures a range of high-performance glasses for technical applications. To find out more about our fused silica and porous silica products, or to discuss a custom glass application, get in touch with a member of our team today.
 |
| Beginning of heat input |
 |
| Progress of heat input showing some parts of the base are compeletly heated while others are not |
 |
| At the beginning of the cool the heat loss is from the surface and to a lesser extent through the shelf. |
|
|
Further information is available in the ebook Low Temperature Kiln Forming. |
Posted Krista Grayson on Dec 3, 2020

Certain types of glass offer excellent shielding against various types of radiation, making them indispensable for applications in medicine and the nuclear industry. In this article, we take a look at some of the most common applications of radiation-shielding glass, and the ways in which glass can be modified to shield against radiation.
Discovery of x-rays by Roentgen in 1895 was followed by a flurry of x-ray research: around 1000 x-ray research papers were published within a single year. Unfortunately, scientists of the time were late to establish that x-rays were capable of damaging living tissue. By the end of 1896, numerous cases of x-ray dermatitis and more serious conditions were reported within the scientific community.1
Though the risks of radiation exposure were not quickly accepted, radiation shielding equipment was developed as early as 1896. Many of these early interventions made use of lead glass to absorb radiation: These included lead glass backings for fluorescent screens, and thick lead glass goggles to protect against cataracts.
Today, lead glass and other types of specialized glass are considered vital materials for protection against radiation exposure. As well as offering tunable mechanical, chemical and optical properties, glasses that contain lead strongly absorb gamma, x-ray, and neutron radiation. This unique set of properties makes glass an invaluable radiation shield for applications where line-of-sight is required, such as in medical radiography and nuclear fuel processing.
In many of these applications, radiation-shielding glass finds use in the form of containers known as hot cells and gloveboxes. Both are shielded containers with radiation-proof glass viewing windows, used for the safe storage and manipulation of radioactive materials. Hot cells are more thoroughly shielded heavy-duty containers used for high-intensity radiation sources such as spent nuclear fuel rods. Gloveboxes are used for lower intensity radiation sources such as certain radiopharmaceuticals.
In other areas, screens and windows made from radiation-shielding glass protect healthcare workers and researchers from x-ray sources such as spectrometers and computed tomography (CT) scanners.
In general, glasses used for radiation-shielding applications include heavy metal oxide (HMO) modifiers such as lead oxide (PbO) and bismuth oxide (Bi2O3). These chemicals can turn ordinary silicate glass into transparent radiation shields capable of effectively absorbing neutrons, gamma rays and x-rays. The resulting glasses are capable of attenuating radiation at levels comparable to concrete and other standard shielding materials while allowing visible light to pass through.2 Crucially, HMO glasses experience relatively little optical or mechanical degradation as a result of exposure to radiation.
While glasses containing lead oxide are common, increasing the lead content leads to a reduction in both melting point and hardness of the glass.2 This, along with environmental concerns with the use of lead, has encouraged research into other types of HMO glass for radiation shielding applications. These include oxides of boron, tellurium, barium, and silicon.3,4 Some research suggests that these glasses may be able to replace conventional concretes as gamma-ray shielding materials.
One key application of radiation-shielding glass is in nuclear medicine. Radioactive sources or materials are used either for imaging purposes (such as positron emission tomography (PET) scans) or therapeutic use (such as radiation therapy). Hot cells and gloveboxes are widely used in the preparation of radiopharmaceuticals, where they allow personnel to process radioactive substances without exposure to dangerous amounts of radiation. Material handling is achieved with the use of remote manipulators or shielded gloves, while radiation-shielded glass windows allow personnel to see inside.
Radiographers are also at risk of exposure to harmful radiation. While scans such as x-rays are generally considered acceptably safe for use in the diagnosis of medical conditions, radiographers carrying out multiple scans per day rely on radiation shielding to minimize their exposure to radiation. Leaded glass windows can strongly absorb both x-rays and gamma rays, allowing radiographers to oversee x-ray or PET scans without exposing themselves to harmful levels of radiation.
Effective radiation shielding is of paramount importance throughout the nuclear industry. Nuclear reactors, spent fuel rods, and fission byproducts all produce many types of harmful radiation in large quantities. Some of these types of radiation are more easily shielded than others: for example, alpha and beta radiation are easily shielded by a thin layer of aluminum or acrylic. However, other radiation types such as gamma, x-ray, and neutron emission can be more challenging to protect against.
Typically, these types of radiation are attenuated by thick concrete shielding. However, in waste reprocessing and laboratory applications, windows of radiation-shielding glass can be used to enable workers to safely view radioactive materials during processing.
Radiation-shielding glass is used in many other applications throughout research and industry, for example in cyclotron maintenance, non-destructive materials testing, and the construction of airport x-ray machines.5 Glass is also used for radiation shielding in space technology for protecting both humans and equipment from cosmic rays — an application for which Mo-Sci is currently developing a lightweight radiation-shielding glass.
Posted Krista Grayson on Oct 29, 2020

Controlled pore glass (CPG) is a high-silica glass that contains pores with a specific size distribution. Porous glasses can be made into a wide range of geometric forms (such as frit, rods, plates, beads, and hollow spheres), and pore sizes can be precisely tuned from the range of angstroms to millimeters. Controlling pore size means that the physical and chemical reactivity of the glass with gases and liquids can be tailored to specific applications such as chromatography, sensing, and filtering.
In addition to this, porous glasses exhibit high mechanical strength, chemical durability, and thermal stability; which make them superior to other porous media (such as polymers and ceramics) for a variety of applications.1
This article covers how porous glass is made, how pore size can be controlled, and some of the varied applications of this unique material.
Porous glass can be manufactured via several different routes, each of which produces different characteristic pore structures. The most common methods involve phase separation or immiscibility of alkali borosilicate glass.
Alkali borosilicate glass systems consist of a silica glass-former with borate and alkali-oxide additives used to lower the melting temperature of the mixture and impart other properties. In other terms, alkali borosilicate systems are mixtures consisting of the chemical species SiO2, B2O3, and R2O; where R is sodium, potassium, or lithium.
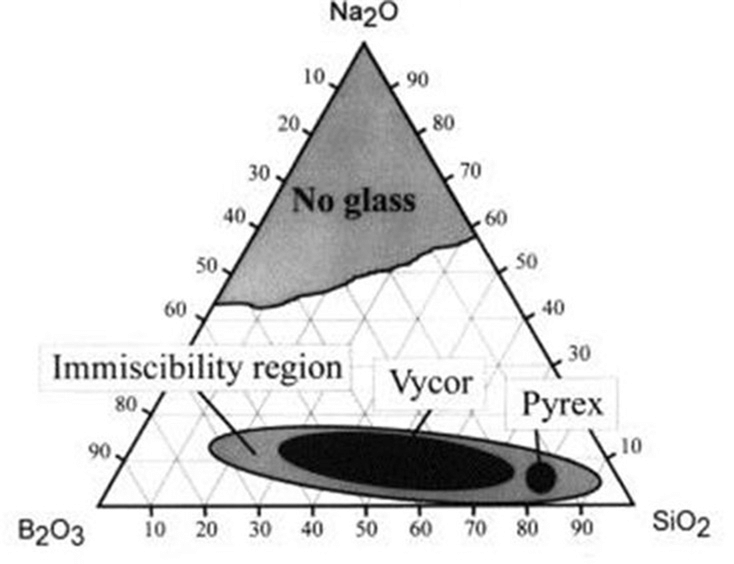
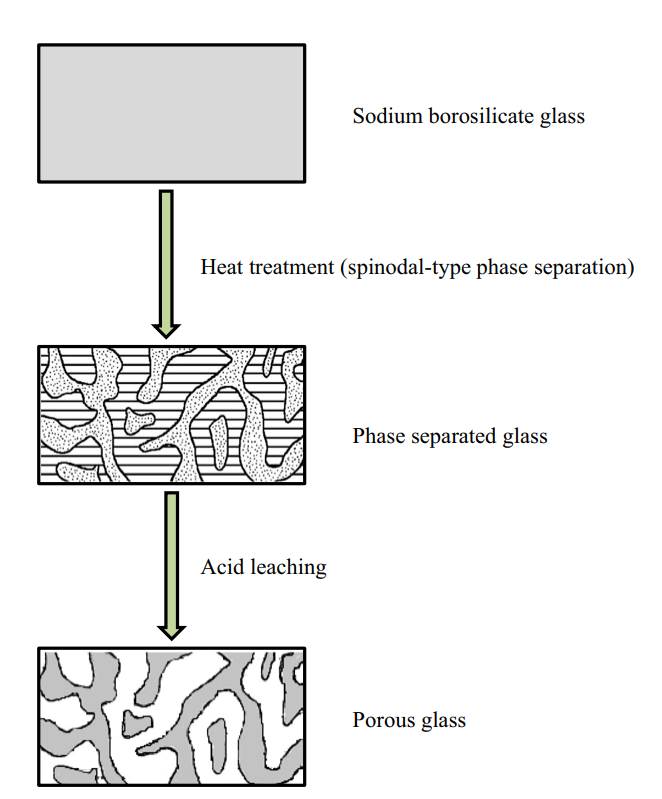
When the constituents of this mixture are tuned to specific concentrations and heated, the entire mixture undergoes an amorphous phase separation: the mixture transforms into two distinct phases.
One of these phases is an alkali-rich borate phase and the other a silica-rich glassy phase. Crucially, the borate phase is soluble in acid, while the silica phase is not. This means that, following heat treatment, the borate phase can be leached out with a hot acid solution. What remains is a highly pure and porous silica glass skeleton with large surface area: in other words, porous glass.
Acid-leaching of a phase-separated mixture generally results in a very narrow pore size distribution, earning the name “controlled-pore glass” and lending the resulting glasses to applications such as adsorptive chromatography of biomolecules.2
The average pore diameter is a function of heat treatment temperature and time, as well as glass composition. Thus, controlling the heat treatment temperature or time (or both) can easily produce porous glasses with a range of pore sizes to suit different applications. Glasses formed via these methods generally have pore diameters in the region of 1 to 1000 nm.3,4
Formation of porous glass using alkali borate systems can also be achieved without inducing a high-temperature phase separation: directly etching the surface of the glass can result in the formation of small pores (1-2 nm) restricted to the surface of the glass.
Porous glass can also be manufactured by glass sintering or via sol-gel routes. Glass sintering is widely used to produce glass foams with pore diameters in the region of 400 m to 1 mm. In sol-gel processes, a solution of organic monomers (sol) is turned into a glass by removal of the liquid phase. Sol-gel processes have been used successfully to create a range of pore sizes for different applications5,6 and they are becoming more common methods.
Porous glass provides an alternative to fused quartz which is comparatively difficult to produce and form into different geometries. However, many emerging applications make use of the functionality offered by the pores themselves. The high surface area and tailorable pore size distribution of these glasses make porous silica a highly effective filtering material, capable of separating not only the basis of molecular size but also of molecule type.7 This, along with a wide range of possible geometries, has made them useful in biosciences and chemistry.1
For example:
All of these applications are made possible by the tunability of pore size, which enables specific physical properties to be imparted in the glass during the manufacturing process.
Mo-Sci produces high purity (> 98% SiO2 and < 2% B2O3) porous glass frit and spheres suitable for applications in industry and research. Contact us to speak with one of our experts about your project requirements.