What
is Viscosity?
 |
| An example of differing viscosities |
There
are a variety of definitions, but these two capture the main
elements.
Informally,
viscosity is the quantity that describes a fluid's resistance to
flow. Fluids resist the relative motion of immersed objects through
them as well as to the motion of layers with differing velocities
within them. Source
Viscosity
is a measure of a fluid's resistance to flow. It describes the
internal friction of a moving fluid. A fluid with large viscosity
resists motion because its molecular makeup gives it a lot of
internal friction. A fluid with low viscosity flows easily because
its molecular makeup results in very little friction when it is in
motion. Source
 |
| A demonstration of the resistance of different viscosities of oil to a weight moving through the liquid. |
Almost
all liquids are viscous fluids having viscidity. For example, when
rotating a drum container filled with water on its vertical central
axis, the water that was at rest in the beginning starts moving as it
is dragged by the container’s inside wall and then whirls
completely together with the container as if it were a single rigid
body. This is caused by the force (resistance) generated in the
direction of the flow (movement) on the surfaces of the water and the
container’s inside wall. A fluid that generates this kind of force
is regarded as having viscosity.
Temperature
is a very important factor for measuring viscosity. In fluids, as
temperature goes up, viscosity goes down and vice versa. In the case
of distilled water, if the temperature changes 1 centigrade, it
produces a difference of 2 % to 3 % in viscosity. Source
Viscosity
is the measurement of a fluid's internal resistance to flow. This is
typically designated in units of centipoise or poise but can be
expressed in other acceptable measurements as well. Source
Why
is viscosity important?
“Near
the strain point the expansion increases rapidly and sometimes
erratically.” The links between the molecules has reduced in
strength and so have a lesser role in the forces acting at higher
temperatures. “In those upper ranges – the temperatures where
glasses are formed and re-formed with heat – viscosity is a much
more useful indicator of how glasses will behave.
“The combination of viscosity and COE are what make glasses more or less compatible, i.e., containing stress in amounts low enough to allow them to hold together without breaking at room temperature for extended periods of time under normal circumstances.
“The combination of viscosity and COE are what make glasses more or less compatible, i.e., containing stress in amounts low enough to allow them to hold together without breaking at room temperature for extended periods of time under normal circumstances.
Bullseye
found in the early 1980s in their efforts to mix coloured glasses in
streaky colour combinations that the COE could not be used to predict
compatibility. In trying to correct the compatibility of certain
mixed glasses, the closer they brought together the COEs, the more
incompatible became the mixes.
“The reason that we could not use COE to successfully predict whether a coloured glass would fit the base clear glass was/is because, as the base glass composition is altered with the addition of the necessary oxides to colour it, the viscosity is inevitably changed. This viscosity change causes the coloured glass and the clear base glass to strain themselves in the cooling cycle of the fusing process (a viscosity mismatch). Therefore once the two glasses reach room temperature they have undue residual strain that may lead to failure.
“In order to prevent this undue residual strain an equal but opposite strain must be introduced into the coloured glass to cancel out the strain induced by the viscosity mismatch. This is accomplished by introducing an expansion mismatch of equal but opposite strain. The two mismatches cancel each other out, leaving the two glasses nearly strain free.
“It is this phenomenon (viscosity mismatch cancelled out by an equal but opposite expansion mismatch) that enables glasses of very different compositions to be formulated to fit each other. The very fact that the expansion of a coloured glass has to be altered to make it fit a base clear glass implies that COE cannot be used as an indicator of compatibility. It is also why it only makes sense to describe these glasses as tested compatible to a specific manufacturer's base glass for a specific glass forming process.“ [L. MacGreggor]
“The reason that we could not use COE to successfully predict whether a coloured glass would fit the base clear glass was/is because, as the base glass composition is altered with the addition of the necessary oxides to colour it, the viscosity is inevitably changed. This viscosity change causes the coloured glass and the clear base glass to strain themselves in the cooling cycle of the fusing process (a viscosity mismatch). Therefore once the two glasses reach room temperature they have undue residual strain that may lead to failure.
“In order to prevent this undue residual strain an equal but opposite strain must be introduced into the coloured glass to cancel out the strain induced by the viscosity mismatch. This is accomplished by introducing an expansion mismatch of equal but opposite strain. The two mismatches cancel each other out, leaving the two glasses nearly strain free.
“It is this phenomenon (viscosity mismatch cancelled out by an equal but opposite expansion mismatch) that enables glasses of very different compositions to be formulated to fit each other. The very fact that the expansion of a coloured glass has to be altered to make it fit a base clear glass implies that COE cannot be used as an indicator of compatibility. It is also why it only makes sense to describe these glasses as tested compatible to a specific manufacturer's base glass for a specific glass forming process.“ [L. MacGreggor]
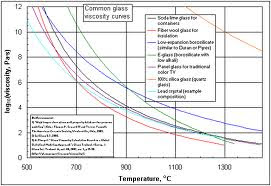 |
| Even different formulations of glass have different viscosities and different rates of softening with temperature increases. |
How
does viscosity apply to us?
Although
viscosity is of major importance to the manufacturer, it does have
some relevance to kiln formers too.
Understanding
that glasses have different viscosities – most often referred to as
hard and soft – can help in the choice of colours and styles of
glass to combine. Some glass will spread more, and also allow other
glass to sink deeper into the layer than others. It might help avoid
combining extremely hard and soft glasses next to each other.
It
should also help explain some results that were not planned. It may
help in when thinking about uneven slumps.
It
is important to recognise that glass chemistry is extremely
complicated, and to see that the expansion characteristics have to be
balanced with the viscosity characteristics as the two main elements
in compatibility. There are others, of course, but these appear to
the two main ones.
No comments:
Post a Comment