Vermiculite
 |
| The screws at the left side of the box are omitted in the drawing, but are required |
 |
| The screws at the left side of the box are omitted in the drawing, but are required |
 |
| The screws at the left side of the box are omitted in the drawing, but are required |
 |
| The screws at the left side of the box are omitted in the drawing, but are required |
Posted by Krista Grayson
While historically a source of problems for glass producers, the phenomenon of phase separation is now known to offer advantages in the production of certain materials such as glass ceramics and porous glasses. Whether desirable or undesirable, understanding and controlling phase separation during the glass manufacturing process is crucial. In this article, we explore the basics of phase separation and how it can be manipulated to create advanced materials for various applications.
In physics and chemistry, the word “phase” refers to a region of a material that is chemically uniform and physically distinct. Phase separation, which typically occurs in liquids, is where a homogeneous mixture separates into two or more of these phases. For example, a mixture of water and oil at room temperature will naturally “phase separate” into a distinct phase consisting of pure oil, and another consisting of pure water. We can say that such a mixture is “immiscible.”
The morphology of this phase separation can vary depending on the relative concentration of both components. If the mixture is predominantly water, the oil phase will take the form of distinct (or “discontinuous”) droplets dispersed throughout an interconnected (or “continuous”) water phase. If the mixture is predominantly oil, the opposite will take place. At roughly equal proportions of oil and water, each phase will tend to be continuous.
Phase separation commonly occurs in glass melts. Borosilicate glass – which contains both silica and borate as network formers – is a well-studied example.1,2
Unlike our water/oil example, phases in glass melts are not necessarily chemically pure. Borosilicate glass, for example, will typically undergo phase separation into a “borate-rich” phase and a “silica-rich” phase, with both phases containing different proportions of each network former. In addition, the morphology of separated phases in glass can vary. While it is possible for droplet-like phases to form via classical nucleation and growth, spontaneous “spinodal” phase separation can result in the formation of intertwined tendril-like continuous phases.3
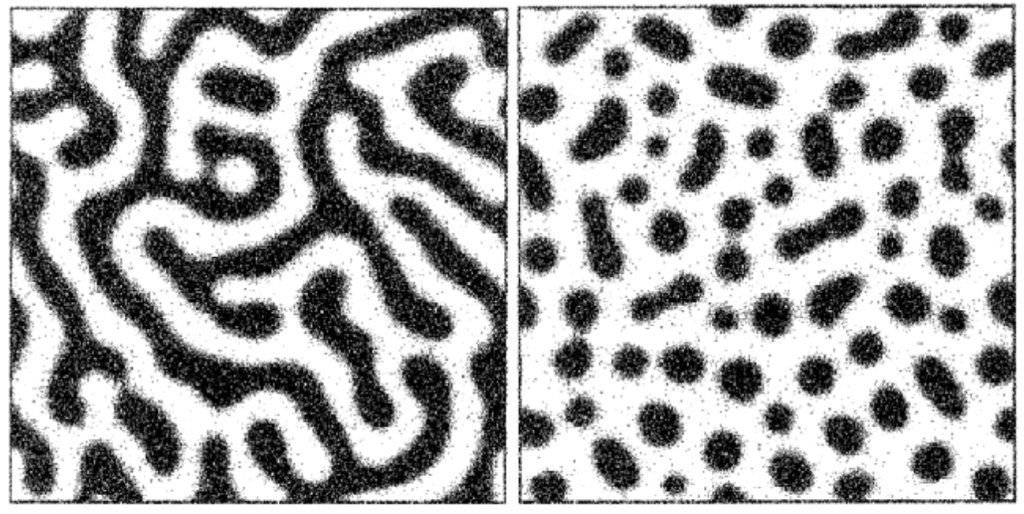
This phase separation, which occurs at high temperatures in the molten glass, persists and “freezes in” when the glass is cooled into a solid. If both phases are vitrifiable, they may form glasses after cooling (this is called a glass-glass phase separation). However, if one phase is prone to crystallization, the mixture can cool into a glass-crystal phase-separated solid.5
Phase separation in glasses was long seen as undesirable – and for many applications, it still is.6 The existence of different phases modifies the physico-chemical properties of glass melts, making it difficult to mold and reduce the quality of the final glass.
The physics of phase separation in glass-forming materials is complex, and even today the specifics are subject to intense debate.7 However, glass manufacturers nonetheless determined ways of avoiding or minimizing phase separation during glass manufacturing.
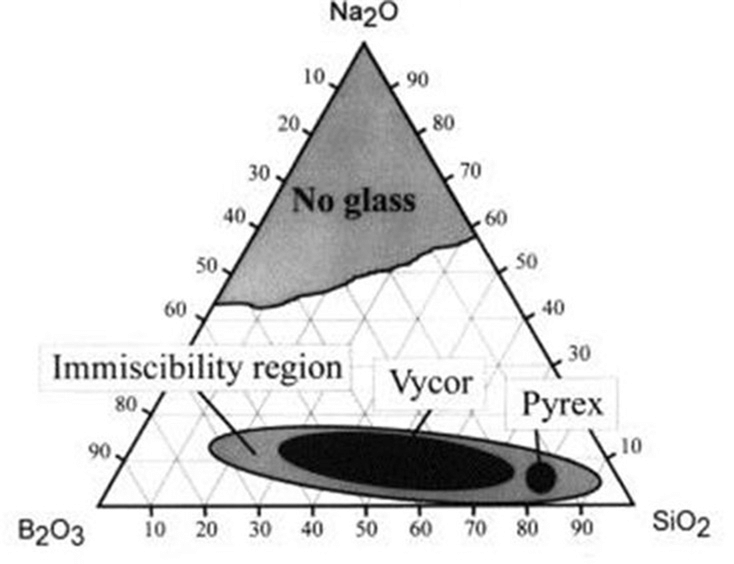
Typically, this is achieved by tailoring the composition of glass melts, with phase separation only occurring for specific compositions. In a Na2O–B2O3–SiO2 glass system, for example, the following ternary phase diagram shows the immiscibility region in which phase separation will occur.
Phase separation (and subsequent crystallization) can also be controlled by the addition of glass modifiers, and by varying heat treatment and cooling rates.9
Note that within the immiscibility region in the diagram above, two common commercial glass compositions are labeled. Indeed, it’s now understood that phase separation offers advantages in certain applications. Today, heterogeneous phase-separated glasses cover a broad range of commercial applications, including Pyrex®, Vycor® opal glass, porous glass, and glass ceramics.
Glass-ceramics are a class of polycrystalline materials that share many properties with both glasses and ceramics, ideally providing the moldability of glasses with various special properties (such as high strength) of ceramics. Glass-ceramics are produced by the formation of crystal phases within an amorphous base glass (i.e., crystal-glass phase separation). Engineering glass-ceramics depends on controlling crystallization within the base material.10
Another application of controlled phase separation is in the production of porous glasses. Porous glasses are typically high-silica glasses that contain pores with a specific size distribution, ranging from angstrom to millimeter scales. Porous glasses are commonly produced from phase separation of alkali borosilicate glass, in which the mixture undergoes spinodal phase separation following heat treatment to yield two continuous phases.11 Following phase separation, the alkali-rich borate phase can be dissolved in acid and removed from the solid. This leaves a highly pure and porous silica glass “skeleton.”
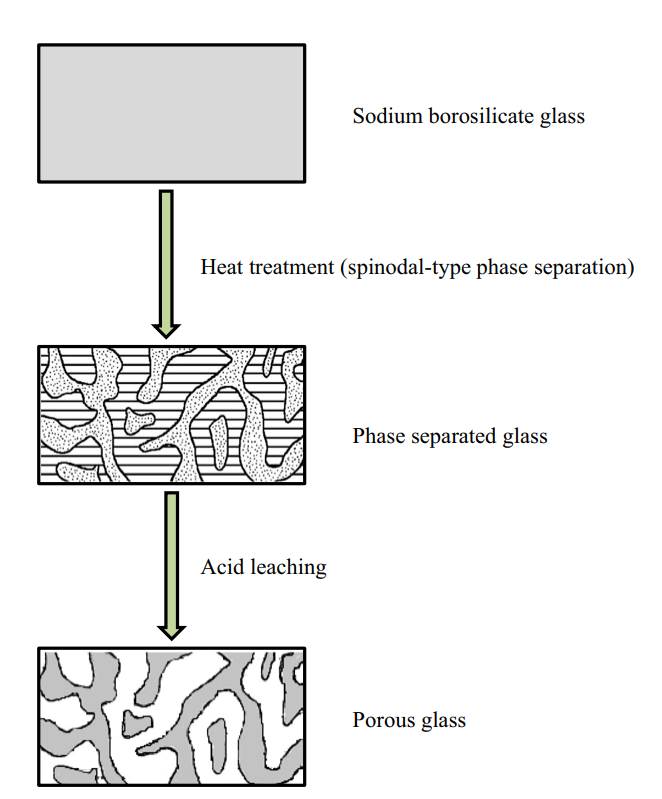
Porous glass exhibits improved mechanical and thermal stability compared to ordinary bulk glass, making it a popular alternative to fused quartz which is comparatively difficult to form. Other applications make use of the pores themselves: such as filitering materials, catalyst supports, and targeted drug delivery.12–16 Mo-Sci is a world-leading provider of advanced glasses for healthcare, electronics and engineering applications. We offer a range of glass-ceramic seals and porous glass solutions, as well as providing custom solutions for virtually any glass application. Contact us for more information.
 Share
Share Tweet
Tweet Share
ShareFuture cities could be 3D printed – using concrete made with recycled glass
From: The Conversation, February 28, 2022 12.39pm GMT
Authors
Associate Professor in Civil Engineering and Environmental Materials, Brunel University London
Marie Skłodowska-Curie Research Fellow, Brunel University London
Associate professor in Civil and Environmental Engineering, West Pomeranian University of Technology in Szczecin
3D printed concrete may lead to a shift in architecture and construction. Because it can be used to produce new shapes and forms that current technologies struggle with, it may change the centuries-old processes and procedures that are still used to construct buildings, resulting in lower costs and saved time.
However, concrete has a significant environmental impact. Vast quantities of natural sand are currently used to meet the world’s insatiable appetite for concrete, at great cost to the environment. In general, the construction industry struggles with sustainability. It creates around 35% of all landfill waste globally.
Our new research suggests a way to curb this impact. We have trialled using recycled glass as a component of concrete for 3D printing.
Concrete is made of a mix of cement, water, and aggregates such as sand. We trialled replacing up to 100% of the aggregate in the mix with glass. Simply put, glass is produced from sand, is easy to recycle, and can be used to make concrete without any complex processing.
Demand from the construction industry could also help ensure glass is recycled. In 2018 in the US only a quarter of glass was recycled, with more than half going to landfill.
We used brown soda-lime beverage glass obtained from a local recycling company. The glass bottles were first crushed using a crushing machine and then the crushed pieces were washed, dried, milled, and sieved. The resulting particles were smaller than a millimetre square.
The crushed glass was then used to make concrete in the same way that sand would be. We used this concrete to 3D print wall elements and prefabricated building blocks that could be fitted together to make a whole building.

If used in this way, waste glass can find a new life as part of a construction material.
The presence of glass does not only solve the problem of waste but also contributes to the development of a concrete with superior properties than that containing natural sand.
The thermal conductivity of soda-lime glass – the most common type of glass, which you find in windows and bottles – is more than three times lower than that of quartz aggregate, which is used extensively in concrete. This means that concrete containing recycled glass has better insulation properties. They could substantially decrease the costs required for cooling or heating during summer or winter.
We also made other changes to the concrete mixture in order to make it more sustainable as a building material, including replacing some of the Portland cement with limestone powder.
Portland cement is a key component of concrete, used to bind the other ingredients together into a mix that will harden. However, the production of ordinary Portland cement leads to the release of significant amounts of carbon dioxide as well as other greenhouse gases. The cement production industry accounts for around 8% of all carbon dioxide emissions in the environment.
Limestone is less hazardous and has less environmental impact during the its production process than Portland cement. It can be used instead of ordinary Portland cement in concrete for 3D printing without a reduction in the quality of the printing mixture.

We also added lightweight fillers, made from tiny hollow thermoplastic spheres, to reduce the density of the concrete. This changed the thermal conductivity of the concrete, reducing it by up to 40% when compared with other concrete used for 3D printing. This further improved the insulation properties of the concrete, and reduced the amount of raw material required.
Using 3D printing technology, we can simply develop a wall structure on a computer, convert it to simple code and send it to a 3D printer to be constructed. 3D printers can operate for 24 hours a day, decrease the amount of waste produced, as well as increase the safety of construction workers.
Our research shows that an ultra-lightweight, well insulated 3D building is possible – something that could be a vital step on our mission towards net zero.